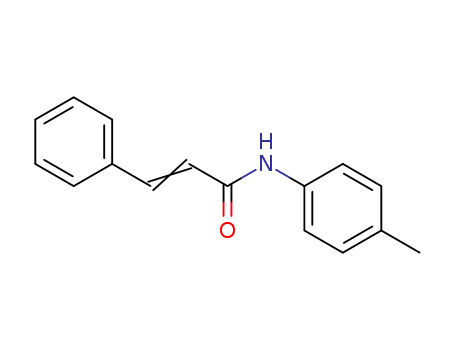
- Chemical Name:N-(4-methylphenyl)-3-phenylacrylamide
- CAS No.:6876-68-2
- Molecular Formula:C16H15NO
- Molecular Weight:237.301
- Hs Code.:
- NSC Number:401976
- Nikkaji Number:J1.339.976J,J2.967.668B
- Pharos Ligand ID:T8KD2WAQ2R32
- ChEMBL ID:CHEMBL2336359
- Mol file:6876-68-2.mol
Synonyms:N-(4-methylphenyl)-3-phenylacrylamide;134430-88-9;N-(p-Tolyl)cinnamamide;(2E)-N-(4-methylphenyl)-3-phenylprop-2-enamide;N-P-Tolylcinnamamide;(E)-N-(4-methylphenyl)-3-phenylprop-2-enamide;6876-68-2;CHEMBL2336359;p-Cinnamotoluidide;NSC401976;SCHEMBL9724353;SMSF0003991;BDBM50429758;MFCD00445047;STK087921;AKOS000487938;CB15721;CCG-357111;NSC-401976;AM805709;AS-63297;BIM-0012401.P001;CS-0037706;SR-01000197333;SR-01000197333-1;5160-05-4