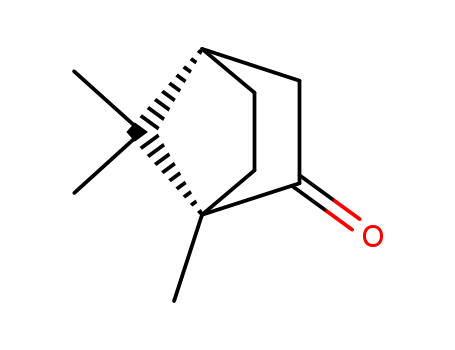
- Chemical Name:Camphor
- CAS No.:76-22-2
- Deprecated CAS:21368-68-3,8013-55-6,8022-77-3
- Molecular Formula:C10H16O
- Molecular Weight:152.236
- Hs Code.:2914.21
- European Community (EC) Number:200-945-0,244-350-4,616-922-7
- ICSC Number:1021
- UN Number:2717
- DSSTox Substance ID:DTXSID5030955
- Nikkaji Number:J4.364H
- Wikipedia:Camphor
- Wikidata:Q181559
- NCI Thesaurus Code:C28136
- RXCUI:1371994
- Pharos Ligand ID:2Q163MUXKRT5
- Metabolomics Workbench ID:75127
- ChEMBL ID:CHEMBL15768
- Mol file:76-22-2.mol
Synonyms:Camphor;Camphor, (+-)-Isomer;Camphor, (1R)-Isomer;Camphor, (1S)-Isomer


 F,
F,  Xn,
Xn,  Xi
Xi
 F:Flammable;
F:Flammable;
