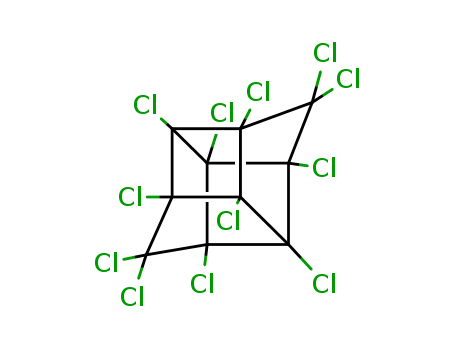
- Chemical Name:Mirex
- CAS No.:2385-85-5
- Deprecated CAS:12557-88-9,12707-43-6,12766-04-0,56449-78-6,20594-49-4,12707-43-6,12766-04-0,20594-49-4,56449-78-6
- Molecular Formula:C10Cl12
- Molecular Weight:545.546
- Hs Code.:2903890030
- European Community (EC) Number:219-196-6
- NSC Number:124102,37656,26107
- UN Number:2761
- UNII:Z917AN264P
- DSSTox Substance ID:DTXSID7020895
- Nikkaji Number:J8.588J
- Wikipedia:Mirex
- Wikidata:Q419578
- NCI Thesaurus Code:C44404
- Metabolomics Workbench ID:74830
- ChEMBL ID:CHEMBL442231
- Mol file:2385-85-5.mol
Synonyms:Mirex