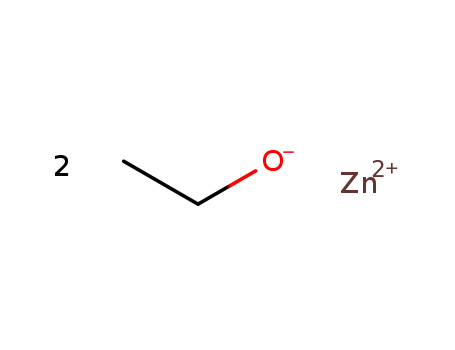
- Chemical Name:Zinc diethanolate
- CAS No.:3851-22-7
- Molecular Formula:C2H6 O . 1/2 Zn
- Molecular Weight:155.512
- Hs Code.:
- European Community (EC) Number:223-355-5
- DSSTox Substance ID:DTXSID60191842
- Nikkaji Number:J217.716A
- Mol file:3851-22-7.mol
Synonyms:Zinc diethanolate;zinc;ethanolate;3851-22-7;EINECS 223-355-5;Diethoxyzinc;SCHEMBL489795;C2H6O.1/2Zn;C2-H6-O.1/2Zn;DTXSID60191842