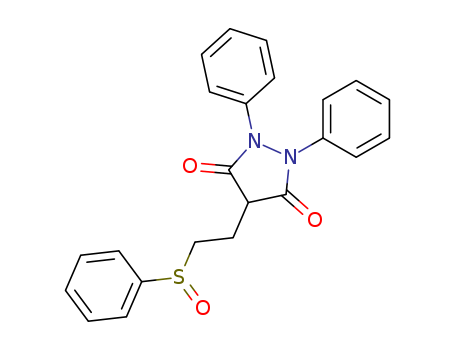
- Chemical Name:Sulfinpyrazone
- CAS No.:57-96-5
- Molecular Formula:C23H20N2O3S
- Molecular Weight:404.489
- Hs Code.:2933194350
- European Community (EC) Number:200-357-4
- NSC Number:757332,75925
- UNII:V6OFU47K3W
- DSSTox Substance ID:DTXSID0023618
- Nikkaji Number:J1.384F
- Wikipedia:Sulfinpyrazone
- Wikidata:Q3790542
- NCI Thesaurus Code:C47739
- Pharos Ligand ID:H1KWLARWYZ5V
- Metabolomics Workbench ID:43360
- ChEMBL ID:CHEMBL832
- Mol file:57-96-5.mol
Synonyms:Anturan;Anturane;Apo Sulfinpyrazone;Apo-Sulfinpyrazone;Nu Sulfinpyrazone;Nu-Sulfinpyrazone;Sulfinpyrazone;Sulfoxyphenylpyrazolidin;Sulphinpyrazone


 Xn
Xn


