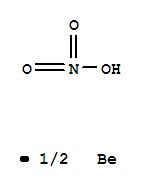Base Information
Edit
- Chemical Name:BERYLLIUM NITRATE
- CAS No.:13597-99-4
- Molecular Formula:Be. 2 H N O3
- Molecular Weight:133.03
- Hs Code.:3822 00 00
- Mol file:13597-99-4.mol
Synonyms:Berylliumnitrate (6CI); Nitric acid, beryllium salt (8CI,9CI); Beryllium dinitrate;Beryllium nitrate (Be(NO3)2)