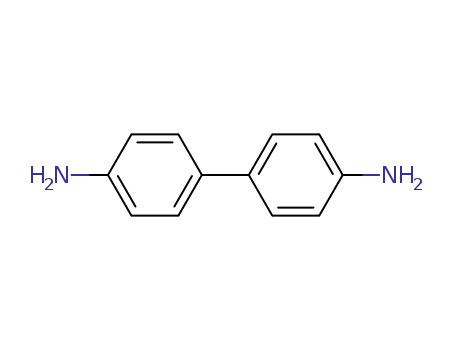
- Chemical Name:Benzidine
- CAS No.:92-87-5
- Deprecated CAS:46310-07-0,56481-94-8,56481-94-8
- Molecular Formula:C12H12N2
- Molecular Weight:184.241
- Hs Code.:29215900
- European Community (EC) Number:202-199-1
- ICSC Number:0224
- NSC Number:146476
- UN Number:1885
- UNII:2X02101HVF
- DSSTox Substance ID:DTXSID2020137
- Nikkaji Number:J3.933K
- Wikipedia:Benzidine
- Wikidata:Q410066
- NCI Thesaurus Code:C44335
- Metabolomics Workbench ID:49570
- ChEMBL ID:CHEMBL15901
- Mol file:92-87-5.mol
Synonyms:benzidine;benzidine acetate;benzidine dihydrochloride;benzidine hydrochloride;benzidine monosulfate


 T,
T, N,
N, F,
F, Xn
Xn


