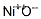1314-06-3 Usage
Chemical Properties
solid
Uses
Different sources of media describe the Uses of 1314-06-3 differently. You can refer to the following data:
1. Storage batteries.
2. Nickel oxide (NiO) is produced from nickel minerals to form nickel oxide when heated to
400°C, which is then reduced at a temperature of 600°C, resulting in the formation of nickel
oxide. It is used as electrodes in fuel cells.
3. Nickel oxide is used in the ceramic industry for making frit, ferrites, and coloring porcelain. The oxide in sinter form is used in the production of nickel-steel alloys. It supplies oxygen to the melt for removal of carbon as carbon dioxide. Some other important uses of nickel oxide include preparation of many nickel salts, specialty chemicals, and nickel catalysts. It also is used as an electrode in fuel cells.
4. ▼▲
Industry
Application
Role/benefit
Chemical manufacture
Manufacture of nickel salts and other nickle compounds
Source of nickel
Manufacture of metallic nickel
Manufacture of nickel steel alloys
Ceramic pigments
Coloring of ceramics and glass
NiO-based black ceramic pigments
Electrical cell
Nickel-iron battery
Active material of negative electrode
Ni-Cd rechargeable batteries
Ni-MH battery
Active material of positive electrode
Tandem solar cell
Photocathodes material
Electronic
Electrochromic devices
Anodic electrochromic material
Others
Hydrogenation reaction
Catalyst
Hazard
Confirmed carcinogen.
Industrial uses
Nickel produces a bluish-violet in potash glasses and a violet tending toward brown in soda glasses. Nickel rates as one of the more powerful colorants, since 1 part in 50,000 produces a recognizable tint. Nickel oxide is sometimes used to decolorize potash glass. Nickel oxide and nickel silicate have an advantage over manganese dioxide for decolorizing purposes in that they are not as sensitive in changing oxidizing and reducing environments.
Safety Profile
Confirmed human
carcinogen. Poison by subcutaneous route.
Mutation data reported. Hazardous reaction
with hydrogen peroxide. Presence of the
oxide increases the sensitivity of
nitroalkanes (e.g., nitromethane, nitroethane,
1 -nitropropane) to heat. See also NICKEL
COMPOUNDS and PEROXIDES.
Check Digit Verification of cas no
The CAS Registry Mumber 1314-06-3 includes 7 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 4 digits, 1,3,1 and 4 respectively; the second part has 2 digits, 0 and 6 respectively.
Calculate Digit Verification of CAS Registry Number 1314-06:
(6*1)+(5*3)+(4*1)+(3*4)+(2*0)+(1*6)=43
43 % 10 = 3
So 1314-06-3 is a valid CAS Registry Number.