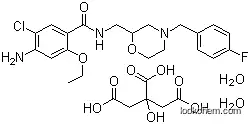
156925-25-6Relevant articles and documents
Synthesis method of mosapride citrate
-
, (2020/07/15)
The invention provides a preparation method of mosapride citrate, namely 4-amino-5-chloro-2-ethoxy-N-((4-(4-fluorophenyl)morpholin-2-yl)methyl)benzamide citrate. The method provided by the invention has the advantages of cheap and easily available raw materials, short reaction steps, high yield, simple post-treatment and the like, reduces the cost, has certain technical advantages, and is suitablefor large-scale industrial production.
METHODS FOR PREPARING MOSAPRIDE CITRATE HYDRATE AND PHARMACEUTICAL COMPOSITION COMPRISING THE SAME
-
Paragraph 0121-0145, (2020/10/19)
The present invention relates to a method for preparing a salt hydrate of mosapride and a pharmaceutical formulation comprising the same. The present invention relates to a method for manufacturing a semiconductor device. A pharmaceutical composition for controlled release and a pharmaceutical composition containing the same are provided to have little side effect, little side effect with other drugs or excipients, and have excellent physical properties for use in a pharmaceutical composition containing the controlled release pharmaceutical composition. (by machine translation)
Citric acid mosapride intermediate product and application
-
, (2018/09/08)
The invention belongs to the field of medical chemistry synthesis, and provides a preparation method of citric acid mosapride intermediate product IV 4-[(4-fluorophenyl)methyl]-2-morpholinemethanaminesalt and citric acid mosapride. The 2-(4-fluorobenzoamido)ethanol and 1H-Isoindole-1,3(2H)-dione,2-(2-oxiranylmethyl) are taken as raw materials, and the intermediate product IV 4-[(4-fluorophenyl)methyl]-2-morpholinemethanamine salt is obtained after acid treating is conducted; the intermediate product IV and an intermediate V 2-oxethyl-4-acetamido-5-Chlorobenzoic acid ethyl ester compounds aretaken as raw materials, dichloromethane is taken as a solvent, and EDCI and DMAP are taken as catalysts to prepare mosapride salt; the mosapride salt is reacted with citric acid aqueous solution to prepare citric acid mosapride. The intermediate product has the advantages that products are high in yield, raw materials are easy to obtain, the production cost is low, and the intermediate product issuitable for industrialized production.
4-amino-5-chloro-2-ethoxy-N- "[ 4-(4-Flurobenzyl)-2-morphorinyl] methyl" phenylbenzamide hydroxycitric salt 2 hydrate production method
-
Paragraph 0065; 0069; 0071, (2016/10/08)
PROBLEM TO BE SOLVED: To provide a method for producing a high-purity 4-amino-5-chloro-2-ethoxy-N-[[4-(4-fluorobenzyl)-2-morpholinyl]methyl]benzamide citric acid salt dehydrate, which produces less by-product. SOLUTION: The method for producing a 4-amino-5-chloro-2-ethoxy-N-[[4-(4-fluorobenzyl)-2-morpholinyl]methyl]benzamide citric acid salt dehydrate includes reacting 4-amino-5-chloro-2-ethoxy-N-[[4-(4-fluorobenzyl)-2-morpholinyl]methyl]benzamide and citric acid at ≥30°C and ≤70°C in a mixed solvent of water and a water-soluble organic solvent. COPYRIGHT: (C)2012,JPOandINPIT
HIGHLY PURE MOSAPRIDE CITRATE DIHYDRATE AND PROCESSES FOR ITS PREPARATION
-
Page/Page column 11, (2011/10/03)
The present invention provides for highly pure mosapride citrate dihydrate and processes for its preparation. The present invention further provides a process for the preparation of mosapride citrate dihydrate substantially free of impurity D-II.
PROCESS FOR THE SYNTHESIS OF A BENZAMIDE DERIVATIVE
-
Page 9-10, (2010/02/04)
The invention relates to a process for the synthesis of mosapride citrate of formula (I), the chemical name: (R,S)-4-amino-5-chloro-2-ethoxy-N-{ [4-(4-fluoro-benzyl)-2- morpholinyl]-methyl}benzamide citrate dihydrate, (I), (II) reacting the compound of formula (II) with di-tert-butyl-dicarbonate in an alcohol in the presence of a base, the obtained product is ethylated in an inert solvent in the presence of a base, the obtained compound is hydrolyzed with an alkyl-hydroxide and the obtained salt neutralized with an acid, the obtained product is chlorinated, and the obtained compound of formula (VI) is reacted with the compound of formula (VII), (VI), (VII) where BOC is tert-butoxy-carbonyl protecting group and removing the protecting group from the obtained compound of formula (VIII) the mosapride base is prepared, (VIII) where BOC is defined as above and in desired case with an acid, preferably with citric acid a pharmaceutically acceptable salt, preferably the mosapride citrate dehydrate of formula (I) is produced.