203120-17-6 Usage
Uses
Used in Pharmaceutical Industry:
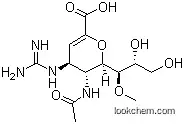
(4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid is used as a potent neuraminidase (NA) inhibitor for the treatment of influenza virus infections. Its application is based on its ability to inhibit the NA enzyme, which is essential for the replication and spread of the influenza virus. (4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid has demonstrated long-acting anti-influenza virus activity, making it a valuable addition to the arsenal of antiviral drugs.
Used in Diagnostic Industry:
(4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid is also used as a potent labeled neuraminidase, which can be utilized in the development of diagnostic tools for detecting and monitoring influenza virus infections. The labeled neuraminidase can help researchers and healthcare professionals better understand the progression of the infection and the effectiveness of treatment strategies.
Used in Research and Development:
In addition to its applications in the pharmaceutical and diagnostic industries, (4S,5R,6R)-5-Acetamido-4-guanidino-6-((1R,2R)-2,3-dihydroxy-1-methoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid can be used as a research tool for studying the structure-activity relationships of neuraminidase inhibitors. This knowledge can be applied to the design and development of new and more effective antiviral drugs targeting the influenza virus.
Originator
Sankyo Co., Ltd. (Japan)
Synthesis
Laninamivir octanoate is prepared starting from a neuraminic acid precursor. The route from 2,3-didehydroneuramic acid entails a multistep sequence to protect the acid and hydroxyl groups at the 4-, 20-, and 30-positions. Methylation of the remaining 10-hydroxyl by treatment with dimethylsulfate and NaH is followed by conversion of the 4-hydroxyl to an amine Cleavage of the 20,30-dihydroxy protecting group, conversion of the 4- NH2 to the guanidine, and acylation of the 30-OH group afford laninamivir octanoate. This three-step sequence can be reordered such that the guanidine is introduced first, followed by deprotection of the 20,30-diOH groups and acylation. An alternative sequence involves a Boc-protected guanidine intermediate, which is converted in a four-step sequence (deprotection of the acid and 20,30-hydroxyl groups, reprotection of the acid as its diphenylmethyl ether, acylation of the 30-OH and deprotection of the guanidine group) to laninamivir octanoate. Laninamivir can also be synthesized from the a-methyl glycoside of N-acetylneuramic acid methyl ester by an analogous route.
Check Digit Verification of cas no
The CAS Registry Mumber 203120-17-6 includes 9 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 6 digits, 2,0,3,1,2 and 0 respectively; the second part has 2 digits, 1 and 7 respectively.
Calculate Digit Verification of CAS Registry Number 203120-17:
(8*2)+(7*0)+(6*3)+(5*1)+(4*2)+(3*0)+(2*1)+(1*7)=56
56 % 10 = 6
So 203120-17-6 is a valid CAS Registry Number.
InChI:InChI=1/C13H22N4O7/c1-5(19)16-9-6(17-13(14)15)3-8(12(21)22)24-11(9)10(23-2)7(20)4-18/h3,6-7,9-11,18,20H,4H2,1-2H3,(H,16,19)(H,21,22)(H4,14,15,17)/t6-,7+,9+,10+,11+/m0/s1
203120-17-6Relevant articles and documents
Synthesis and anti-influenza evaluation of polyvalent sialidase inhibitors bearing 4-guanidino-Neu5Ac2en derivatives.
Masuda, Takeshi,Yoshida, Shuku,Arai, Masami,Kaneko, Satoru,Yamashita, Makoto,Honda, Takeshi
, p. 1386 - 1398 (2003)
Polyvalent sialidase inhibitors bearing 4-guanidino-Neu5Ac2en derivatives on a poly-L-glutamine backbone are described. Aiming for a longer retention time of 4-guanidino-Neu5Ac2en (zanamivir) in bronchi and lungs, we focused on supermolecules bearing 4-gu
METHOD FOR PRODUCING NEURAMINIC ACID DERIVATIVE
-
Paragraph 0141, (2014/10/29)
The present invention provides methods for manufacturing neuraminic acid derivatives. [Means for solution] Methods for manufacturing compounds represented by the formula (I) : [wherein R1 represents a C1-C19 alkyl group], or a pharmacologically acceptable salt thereof, using N-acetylneuraminic acid dihydrate as a starting raw material are provided.
Organocatalytic and scalable synthesis of the anti-influenza drugs zanamivir, laninamivir, and CS-8958
Tian, Junshan,Zhong, Jiankang,Li, Yunsheng,Ma, Dawei
, p. 13885 - 13888 (2015/04/16)
Zanamivir, laninamivir, and CS-8958 are three neuraminidase inhibitors that have been clinically used to combat influenza. We report herein a novel organocatalytic route for preparing these agents. Only 13 steps are needed for the assembly of zanamivir and laninamivir from inexpensive d-araboascorbic acid by this synthetic route, which relies heavily on a thiourea-catalyzed enantioselective Michael addition of acetone to tert-butyl (2-nitrovinyl)carbamate and an anti-selective Henry reaction of the resulting Michael adduct with an aldehyde prepared from d-araboascorbic acid. The synthetic procedures are scalable, as evident from the preparation of more than 3.5 g of zanamivir.
DRUG FOR TREATMENT OF INFLUENZA
-
Page/Page column 9, (2009/12/07)
A therapeutic or prophylactic for H5N1 influenza is provided. Specifically disclosed is a therapeutic or prophylactic for H5N1 influenza comprising as an active ingredient a compound represented by the general formula (I): wherein R1 and R2 represent H or alkanoyl; X represents a halogen atom, OH, alkoxy or alkanoyloxy; and R3 represents H or alkyl; PROVIDED THAT compounds of formula (I) wherein each of R1 and R2 is H, X is OH, and R3 is H are excluded.
METHOD FOR MANUFACTURING NEURAMINIC ACID DERIVATIVES
-
Page/Page column 77, (2008/12/08)
A method for manufacturing neuraminic acid derivatives is provided, also synthetic intermediates of the neuraminic acid derivatives and methods for their manufacture, and neuraminic acid derivatives having high purity. [Means for solution] A synthetic intermediate compound represented by the formula (7) is provided: [wherein R3 represents alkyl; R4 and R5 each represents H, alkyl, phenyl, or together represent tetramethylene, pentamethylene, oxo].