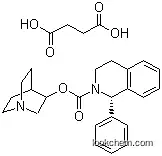
242478-38-2Relevant articles and documents
Solifenacin succinate new preparation method
-
Paragraph 0012; 0014, (2018/08/03)
The present invention relates to a solifenacin succinate new preparation method which mainly comprises the following steps: 1) formula III compound (R)-(-)-quinuclidin-3-ol and formula IV compound N,N,-N,N-carbonyldiimidazole are reacted to obtain formula V compound (R)-imidazole-1-carboxylic acid-1-azabicyclo[2,2,2] octyl ester; 2) the formula V compound (R)-imidazole-1-carboxylic acid-1-azabicyclo[2,2,2] octyl ester is reacted with formula VI compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline to obtain a formula II solifenacin alkaline body; 3) the formula II solifenacin alkaline body isreacted with succinic acid, refined and purified to obtain formula I solifenacin succinate. The preparation method has the advantages that the yield of the solifenacin succinate is high and the purityis high, and the process is simple and suitable for industrial production.
A succinic acid thorley that new preparation method
-
Paragraph 0018; 0037-0039, (2017/09/19)
The invention relates to a preparation method of succinic acid solifenacin, and belongs to the technical field of medicinal raw material preparation. According to the technical scheme, the preparation method of the novel succinic acid solifenacin is characterized in that R-3-quinuclidinol, S-1-phenyl-1, 2, 3, 4-tetrahydro naphthalene are used as raw materials, a reaction product of triphosgene and substituted phosphine oxide is used as a condensing agent, the solifenacin is prepared through a one-pot method, and then the succinic acid solifenacin is obtained through salifying. The preparation method of the succinic acid solifenacin has the beneficial effects that the operation is simple, reaction conditions are mild, and the preparation method is suitable for industrial amplification production.
A process for the preparation of new thorley that succinic acid
-
Paragraph 0071, (2016/10/07)
The invention relates to a method for preparing a solifenacin succinate ((3R)-1-azabicyclo [2.2.2] octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate succinate). The method comprises the following steps: 1) preparing a compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline carboxylic acid ethyl ester of formula III from a compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline, serving as a raw material, of formula IV; 2) synthesizing a compound (3R)-1-azabicyclo [2.2.2] octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate of formula II in an ionic liquid by using the compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline carboxylic acid ethyl ester of formula III; and 3) salifying the compound (3R)-1-azabicyclo [2.2.2] octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate of formula II in an organic solvent to obtain the compound solifenacin succinate ((3R)-1-azabicyclo [2.2.2] octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate succinate) of formula I. The preparation method has the advantages that the process is simple, the method is suitable for industrialized production, the yield of the product is high and the purity of the product is high.
After-treatment for solifenacin and method for preparing succinic acid solifenacin
-
Paragraph 0062; 0063, (2016/12/01)
The invention discloses an after-treatment method for solifenacin. The method comprises the steps that strong acid is slowly dripped into total synthesis liquid of solifenacin, after a pH value is adjusted to be 1.0-2.0, temperature is raised, water is added, and an extracting agent is used for extraction; after a water phase is cooled, a saturated sodium carbonate solution is added to alkalinity, dichloromethane is added to carry out graded extraction, extract liquor is washed with water, and a drying agent is added for drying, so that a dry product is obtained; the dry product is steamed at low temperature to be dry to obtain an oily substance solifenacin. By optimizing the after-treatment technology, isopropyl ether and dichloromethane are only used in the after-treatment technology of solifenacin, types of solvent are reduced, operation and recycle are facilitated, and cost is saved greatly. Strong base or strong acid is not used in the alkali efflorescence process of hydrochloric acid solifenacin, a saturated NaCO3 solution is used, the reaction is mild, temperature can be controlled easily, most importantly, generation of isomers is greatly reduced, and the content of the isomers of a non-structure type (1S and 3R) is reduced.
PROCESS OF PREPARING SOLIFENACIN OR SALT THEREOF, AND NOVEL INTERMEDIATE USED IN THE PROCESS
-
Paragraph 0084 - 0108, (2015/04/28)
Disclosed herein is a method of preparing solifenacin or a salt thereof, including the steps of: (a) reacting (R)-quinuclidinol with bis(pentafluorophenyl)carbonate in an organic solvent to prepare a solifenacin intermediate, (3R)-1-azabicyclo[2,2,2]oct-3-yl pentafluorophenylcarbonate, and (b) reacting the solifenacin intermediate with (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline in an organic solvent to prepare solifenacin. The method is advantageous in that high-purity solifenacin or a salt thereof can be simply and efficiently prepared with high yield using a novel intermediate.
A PROCESS FOR THE PREPARATION OF SOLIFENACIN OR A SALT THEREOF
-
Page/Page column 8, (2014/01/18)
The present invention relates to an improved process for the preparation of Solifenacin or its salt of formula I, more particularly the present invention relates to an economically viable and industrially advantageous process for the preparation of highly pure Solifenacin or its salt of formula I.
Process for the Preparation of Solifenacin and Salts Thereof
-
, (2014/08/19)
The invention provides a new process for the preparation of solifenacin or a pharmaceutically acceptable acid addition salt thereof, comprising reacting (R)-quinuclidin-3-yl phenethylcarbamate with benzaldehyde in the presence of an acid to obtain a diast
An improved process for the preparation of highly pure solifenacin succinate via resolution through diastereomeric crystallisation
Trinadhachari, Ganala Naga,Kamat, Anand Gopalkrishna,Venkata Balaji, Boddu,Prabahar, Koilpillai Joseph,Naidu, Kolukuluru Mohan,Babu, Korupolu Raghu,Sanasi, Paul Douglas
, p. 934 - 940 (2014/10/15)
An improved process for the preparation of solifenacin succinate (1) involving resolution through diastereomeric crystallization is described. (1S)-IQL derivative (5) is esterified to form (1S)-ethoxycarbonyl IQL derivative (6) which is condensed with (RS)-3-quinuclidinol (7) to form a solifenacin diastereomeric mixture (8); this is subjected to resolution through diastereomeric crystallization to produce solifenacin succinate (1), which is used for the treatment of an overactive bladder.
NOVEL PROCESS FOR THE PREPARATION OF SOLIFENACIN SUCCINATE
-
Paragraph 0038; 0039; 0040; 0041, (2013/05/22)
The present invention relates to a process for the preparation of Solifenacin succinate by condensing a compound of formula (IVb) with (RS)-3-quinuclidinol, wherein, R represents methyl, ethyl, isopropyl; to produce a diastereomeric mixture of (1S)-3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylic acid (3RS)-1-azabicyclo[2.2.2]oct-3-yl ester, which is treated with succinic acid in a solvent or mixture of solvents to produce optically pure Solifenacin succinate, Formula (X).
PROCESS OF PREPARING SOLIFENACIN OR SALT THEREOF, AND NOVEL INTERMEDIATE USED IN THE PROCESS
-
Paragraph 147-154, (2013/10/21)
Disclosed herein is a method of preparing solifenacin or a salt thereof, including the steps of: (a) reacting (R)-quinuclidinol with bis(pentafluorophenyl)carbonate in an organic solvent to prepare a solifenacin intermediate,(3R)-1-azabicyclo[2,2,2]oct-3-yl pentafluorophenylcarbonate, and (b) reacting the solifenacin intermediate with (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline in an organic solvent to prepare solifenacin. The method is advantageous in that high-purity solifenacin or a salt thereof can be simply and efficiently prepared with high yield using a novel intermediate.