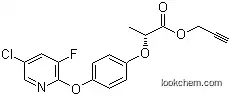
105512-06-9Relevant articles and documents
Synthesis method of clodinafop-propargyl
-
Paragraph 0015-0021, (2021/01/29)
The invention relates to the field of chemical engineering, and discloses a synthesis method of clodinafop-propargyl. The method comprises the following steps of: dissolving DHPPA in an organic solvent, heating to a temperature of 50 DEG C, adding an anti
NOXIOUS ARTHROPOD CONTROL AGENT CONTAINING AMIDE COMPOUND
-
, (2017/08/26)
An object of the present invention is to provide a compound having the controlling activity on a noxious arthropod, and a noxious arthropod controlling agent containing an amide compound of formula (I): wherein X represents a nitrogen atom or a CH group, p represents 0 or 1, A represents a tetrahydrofuranyl group or the like, R1, R2, R3, R4, R5, R6 and R7 represent a hydrogen atom or the like, n represents 1 or 2, Y represents an oxygen atom or the like, m represents any integer of 0 to 7, and Q represents a C1-8 chain hydrocarbon group optionally having a phenyl group or the like, has the excellent noxious arthropod controlling effect.
Preparation method of clodinafop propargyl
-
Paragraph 0038; 0044; 0045; 0046; 0047, (2016/10/17)
The invention relates to a preparation method of clodinafop propargyl used for controlling grassy weeds in wheat fields. R-2-(p-hydroxyphenoxy)propionic acid is taken as a raw material and has a reaction with caustic alkali in water and an aprotic polar s
LIQUID FORMULATIONS
-
, (2011/06/28)
The present invention relates to a liquid formulation comprising a) agrochemically active salts of 2-iodo-N-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoyl]benzenesulfonamide, and b) one or more non-polar organic solvents selected from the group of the C6-C16-aromatics mixture the Solvesso series (Exxon) and/or the Caromax series (Carless), and also ?optionally further non-polar organic solvents. The liquid formulation is suitable for crop protection.
A new method for one-pot synthesis of aryloxyphenoxypropionate herbicides using 2,4,6-trichloro-1,3,5-triazine and (n-BU)4NI as a homogeneous catalyst
Kalhor, Mehdi,Dadras, Akbar,Mobinikhaledi, Akbar,Tajik, Hassan
experimental part, p. 833 - 836 (2012/05/04)
The one-pot reaction of halo-heterocycle, (R)-4-hydroxyphenoxy propionic acid and an alcohol, amine or sulfonamide is described as an efficient method for the synthesis of aryloxyphenoxy propionate hrerbicides by using 2,4,6-trichloro-1,3,5-triazine in the presence of (n-BU) 4NI, as a homogeneous catalyst under mild conditions. The present procedure offers several advantages, such as good yields, short reaction times and easy workup.
A facile synthesis of novel optically active R,R-2-(4-(2-(4-(5-chloro-3- halo-pyridin-2-yloxy)-phenoxy)-propionyloxy)-phenoxy)-propionic acid esters using cyanuric chloride as potential herbicide
Tajik, Hassan,Dadras, Akbar,Aghabeygi, Shokufeh
, p. 535 - 538 (2012/01/05)
A facile method for the synthesis of a new series of R,R-2-(4-(2-(4-(5- chloro-3-halopyridin-2-yloxy)-phenoxy)-propionyloxy)-phenoxy)-propionic acid ester derivatives containing a halo-substituted pyridine carrying two R,R chiral centers from (R)-2-(4-hyd
Solid adjuvants
-
, (2008/06/13)
The present invention relates to a solid adjuvant comprising a) one or more surfactants of the formula (I), Ar—O—(CHR1—CHR2—O—)y—R3??(I) where Ar is aryl which is substituted by at least two (C1-C30)alkyl radicals, R1 is H or (C1-C6)alkyl, R2 is H or (C1-C6)alkyl, R3 is H, an unsubstituted or substituted (C1-C30) hydrocarbon radical, a sulfonate radical, a phosphonate radical or an acyl radical, and y is an integer from 1 to 100, and b) one or more fatty acid esters. The adjuvant is particularly suitable in the field of crop protection.
Herbicidal compositions
-
, (2008/06/13)
The present invention relates to a herbicidal composition comprising a) one or more herbicidal active substances, b) one or more surfactants other than silicone surfactants, and c) one or more humectants. The composition according to the invention is outstandingly suitable for controlling a variety of harmful plants.
Herbicidal composition
-
, (2008/06/13)
The present invention relates to a herbicidal composition, comprising A) one or more compounds of the formula (I) 1in which Hal1 and Hal2 are identical or different halogen atoms, R1 is H, a cation or a C1-C20-carbon-containing radical and B) one or more surfactants, comprising as structural element at least 12 alkylene oxide units.