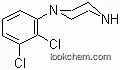
119532-26-2Relevant articles and documents
Synthesis method of cariprazine key intermediate 1-(2,3-dichlorophenyl)piperazine hydrochloride
-
Paragraph 0042-0044; 0051-0056, (2021/11/26)
The invention discloses a synthesis method of a cariprazine key intermediate 1-(2,3-dichlorophenyl)piperazine hydrochloride, and belongs to the field of medicine synthesis. The method comprises the following steps: (1) taking a compound 1 and a compound a as raw materials, and conducting reacting to obtain a compound 2; (2) carrying out nitro reduction reaction by taking the compound 2 and a reducing agent as raw materials to obtain a compound 3; and (3) taking the compound 3 as a raw material, and carrying out diazotization reaction and deprotection reaction to obtain the 1-(2,3-dichlorophenyl)piperazine hydrochloride. The raw materials used in the synthesis method are low in toxicity, low in cost and easy to obtain, no noble metal catalyst or phosphorus ligand is used, the equipment requirement is simple, the product yield is high, the purity is high, and the synthesis method is suitable for large-scale industrial production and has a good application prospect.
Synthesis method of high-purity aripiprazole and preparation method of hydrate particles of aripiprazole
-
Paragraph 0031; 0053; 0131-0134, (2021/08/07)
The invention discloses a synthesis method of high-purity aripiprazole and a preparation method of hydrate particles of aripiprazole. The method comprises the following steps: step (1), carrying out Williamson etherification on 7-hydroxyl-3,4-dihydro-2 (1H)-quinolinone and 1,4-dibromobutane under the action of potassium carbonate to obtain 7-(4-bromobutoxy)-3,4-dihydro-2(1H)-quinolinone; step (2), synthesizing 2,3-dichlorophenyl piperazine hydrochloride from 2,3-dichloroaniline and bis(2-chloroethyl) amine hydrochloride; step (3), carrying out an alkylation coupling reaction of nitrogen on 7-(4-bromobutoxy)-3,4-dihydro-2(1H)-quinolinone and 1-(2,3-dichlorophenyl) piperazine hydrochloride, so as to prepare aripiprazole; (4) refining: recrystallizing aripiprazole by using ethyl acetate to obtain high-purity anhydrous aripiprazole; and (5) preparation of aripiprazole hydrate particles: refluxing and dissolving anhydrous aripiprazole in an ethanol-water system, and controlling the stirring rate and the cooling rate to obtain the aripiprazole hydrate particles.
Compound with dopamine D3 receptor adjusting activity, and applications thereof
-
Paragraph 0191-0194, (2019/03/22)
The invention relates to a compound with dopamine D3 receptor adjusting activity, and applications thereof, and more specifically discloses dopamine receptor D3R as a novel target of post-traumatic stress disorder (PTSD) in prevention, treatment/or auxiliary treatment of PTSD, and screening of anti-PTSD medicines, and relates to applications of a compound with dopamine receptor D3R adjusting activity in preparation of medicines used for preventing, treatment and/or auxiliary treatment of PTSD.
Preparation method of 1-(2,3-dichlorophenyl)piperazine hydrochloride
-
Paragraph 0011; 0012, (2019/06/08)
The invention belongs to the field of preparation of chemical intermediates, and particularly relates to a preparation method of 1-(2,3-dichlorophenyl)piperazine hydrochloride. The preparation methodcomprises the following steps of using diethanol amine as the initial raw material, and performing chlorination reaction, so as to obtain beta,beta'-dichlorodiethylamine hydrochloride; performing cyclization reaction with 2,3-dichloroaniline in a water solution under the condition of no catalyst, so as to synthesize a target compound, namely the 1-(2,3-dichlorophenyl)piperazine hydrochloride. Thepreparation method of the 1-(2,3-dichlorophenyl)piperazine hydrochloride has the advantages that the reaction rate is obviously accelerated, the yield rate is increased, and the cost is reduced.
Deuterated aripiprazole as well as preparation method and application thereof
-
Paragraph 0053; 0060; 0061; 0062, (2018/07/30)
The invention belongs to the fields of organic and medicine synthesis, and discloses deuterated d8 aripiprazole as well as a preparation method and application thereof. According to the preparation method, 1,4-dichlorobutane-d8 serving as a raw material i
Design, synthesis, and evaluation of bitopic arylpiperazine-phthalimides as selective dopamine D3 receptor agonists
Cao, Yongkai,Sun, Ningning,Zhang, Jiumei,Liu, Zhiguo,Tang, Yi-zhe,Wu, Zhengzhi,Kim, Kyeong-Man,Cheon, Seung Hoon
supporting information, p. 1457 - 1465 (2018/10/02)
The dopamine D3 receptor (D3R) is a proven therapeutic target for the treatment of neurological and neuropsychiatric disorders. In particular, D3R-selective ligands that can eliminate side effects associated with dopamine D2 receptor (D2R) therapeutics have been validated. However, the high homology in signaling pathways and the sequence similarity between D2R and D3R have rendered the development of D3R-selective ligands challenging. Herein, we designed and synthesized a series of piperazine-phthalimide bitopic ligands based on a fragment-based and molecular docking inspired design. Compound 9i was identified as the most selective D3R ligand among these bitopic ligands. Its selectivity was improved compared to reference compounds 1 and 2 by 9- and 2-fold, respectively, and it was 21-fold more potent than compound 2. Molecular docking demonstrated that the orientation of Leu2.64 and Phe7.39 and the packing at the junction of helices may affect the specificity for D3R over D2R. Functional evaluation revealed that D3R-selective ligand 9i displayed a subpicomolar agonist activity at D3R with a 199-fold increase in potency compared to quinpirole. These results may be useful for the fragment-based design of bitopic compounds as selective D3R ligands.
ARYLATION OF ALIPHATIC AMINES
-
Page/Page column 27; 29; 30, (2017/09/07)
The invention relates to a method for arylation of amines, such as aliphatic amines by reaction of aryl-halogens, e.g. chloro- or fluorobenzene derivatives without strongly electron withdrawing substituents in the presence of a strong base.
Mechanism and Scope of Base-Controlled Catalyst-Free N-Arylation of Amines with Unactivated Fluorobenzenes
Borch Jacobsen, Christian,Meldal, Morten,Diness, Frederik
supporting information, p. 846 - 851 (2017/02/05)
A general method for transition metal-free N-arylation of amines has been developed. Mechanistic studies have revealed that the ability of the base to facilitate the desired amination without promoting unwanted side reactions is the guiding factor. By employing lithium bis(trimethylsilyl)amide as a base the resultant deprotonated amines readily react with a range of unactivated fluorobenzene derivatives. This new arylation method is utilized for the simple two-step synthesis of the antidepressant Vortioxetine.
Design, synthesis and in vitro activity of 1,4-disubstituted piperazines and piperidines as triple reuptake inhibitors
Paudel, Suresh,Acharya, Srijan,Yoon, Goo,Kim, Kyeong-Man,Cheon, Seung Hoon
, p. 2266 - 2276 (2017/03/23)
Monoamine transporters regulate the concentration of monoamine neurotransmitters, which are essential for vital physiological processes, and their dysfunction can cause several central nervous system diseases. Monoamine transporters currently appear to be the potential target in the management of these disorders. In this study, homologation and bioisosterism techniques have been used in the designing of new 1,4-disubstituted piperazines and piperidines. These derivatives were synthesized and evaluated as potential triple reuptake inhibitors for studying the structure-activity relationships. The most advanced compound, 1-(4-(5-benzhydryl-1H-tetrazol-1-yl)butyl)-4-(3-phenylpropyl)piperazine (2i), was able to inhibit monoamine neurotransmitter reuptake in an in vitro test (IC50?=?158.7?nM for 5-HT, 99?nM for NE and 97.5?nM for DA). These novel potent triple reuptake inhibitor-based 1,4-disubstituted piperazine and piperidine scaffolds deserve further systematic optimization and pharmacological evaluation.