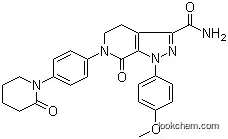
503612-47-3 Usage
Uses
Used in Orthopedic Surgery:
Apixaban is used as an antithrombotic agent for the prevention of venous thromboembolic events (VTE) in adult patients who have undergone elective hip or knee replacement surgery. Its selective inhibition of Factor Xa helps reduce the risk of blood clot formation without inducing adverse bleeding events.
Used in Atrial Fibrillation:
Apixaban is used as an antithrombotic agent for preventing blood clot formation in patients with atrial fibrillation. Its oral bioavailability and selective inhibition of Factor Xa make it an effective treatment option for reducing the risk of stroke and other thromboembolic complications associated with this condition.
Used in Venous Thrombosis:
Apixaban is used as an antithrombotic agent in a rabbit model of venous thrombosis, demonstrating its potential for treating thromboembolic disorders by inhibiting the coagulation cascade and reducing blood clot formation.
Used in Aortic Heterotopic Valve Replacement:
In a porcine model of aortic heterotopic valve replacement, apixaban is used to prevent thrombus formation without causing adverse bleeding events. Its selective inhibition of Factor Xa makes it a valuable option for managing thromboembolic risks in patients undergoing valve replacement surgeries.
Indications and Usage
Apixaban is a new form of oral anticoagulant drug developed by Bristol Myers Squibb and Pfizer. It is a new form of oral Xa factor inhibitor, and its commercial name is Eliquis. Apixaban is used to treat adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolism (VTE)
Mechanisms of Action
Apixaban is an oral selective activated Xa factor inhibitor and can prevent thrombin generation and thrombosis.
Clinical Research
Apixaban is the third new oral anticoagulant to go on sale, following dabigatran and rivaroxaban, and it has already been approved in Europe for preventing venous thromboembolism in patients undergoing elective hip or knee replacement surgery. Out of these three oral anticoagulants approved in Europe, compared to the current standard preventative treatment against venous thromboembolism, enoxaparin, rivaroxaban excelled in the record experiment, and apixaban excelled in the advance experiment. Rivaroxaban’s curative effects were slightly superior, but it caused more severe bleeding than apixaban. Researchers attributed these differences to medication time, as rivaroxaban was taken 6-8 hours after surgery in the record experiment, while apixaban was used 18 hours after surgery in the advance experiment. These drugs have better curative effect when used closer to time of surgery, but also have an increased bleeding risk. Clinical research showed that compared to a daily subdermal injection of 40mg enoxaparin, 2 oral 2.5mg dosages of apixaban had better preventative effects against venous thromboembolism following hip or knee replacement surgery and did not increase bleeding risk.
Drug Interactions
1.A double inhibitor of strong CYP3A4 and P-gp increases apixaban’s blood levels: decrease Eliquis dosage to 2.5mg or avoid simultaneous usage.
2.A inductor of strong CYP3A4 and P-gp can decrease apixaban’s blood levels: avoid simultaneous usage.
Warnings and Precautions
Breastfeeding mothers should stop usage or stop breastfeeding.
Use during pregnancy is not advised.
Use while experiencing severe liver damage is not advised.
Originator
Bristol Myers Squibb Company (United States)
Clinical Use
Apixaban is an oral anticoagulant with highly selective inhibition
of factor Xa. It was approved by the European Medicines
Agency (EMA) for the treatment of venous thromboembolic events
and first marketed in Germany under the brand name Eliquis in
June 2011. Apixaban was co-developed by Bristol-Myers Squibb
and Pfizer and represents the first approved drug for this indication
since warfarin over 50 years ago.
Synthesis
Although several convenient
preparations of apixaban (BMS-562247) have been reported, the most likely process-scale route is described in the scheme. The
starting material 4-iodoaniline (14) was acylated with 5-bromovaleryl
chloride (15) and triethylamine followed by cyclization under
basic conditions to give lactam 16 in 49% yield. Intermediate 16
was then reacted with phosphorus pentachloride to provide the
a,a-dichlorinated lactam 17 in 87% yield.30 This dichloride was reacted
with excess morpholine to affect an alkylation–elimination
sequence to afford enaminolactam 18 in 86% yield. N-Arylation
of this iodide with valerolactam 19 using a copper (I) catalyst resulted
in a 77% yield of the desired p-bispiperidone 20. Interestingly,
sequential exposure of 20 to a nitrile imine generated from
the treatment of ethyl 2-chloro-2-(2,4-methoxyphenyl)-hydrazono)
acetate 21 with base resulted in a [3+2] dipolarcycloadditon
reaction. Upon acidification with 4 N HCl, pyrazole 22 was furnished
in 67% over two steps. Conversion of the ester within 22
to the corresponding amide was achieved via a mixture of formamide
and sodium methoxide to give apixaban (III) in 71% yield. It is
important to note that intermediate 21 was prepared from commercially
available 4-methoxyaniline (23) by sequential diazotization
and condensation with ethyl 2-chloroacetoacetate (24).
in vitro
apixabanhas exhibited a high degree of potency, selectivity, and efficacy on factor xa with ki of 0.08 nm and 0.17 nm for human factor xa and rabbit factor xa, respectively [1]. apixaban prolonged the clotting times of normal human plasma with the concentrations (ec2x) of 3.6, 0.37, 7.4 and 0.4 μm, which are required respectively to double the prothrombin time (pt), modified prothrombin time (mpt), activated partial thromboplastin time (aptt) and heptest. besides, apixaban showed the highest potency in human and rabbit plasma, but less potency in rat and dog plasma in both the pt and aptt assays [3].
in vivo
apixaban exihibited the excellent pharmacokinetics with very low clearance (cl: 0.02 l kg-1h-1), and low volume of distribution (vdss: 0.2 l/kg) in the dog. besides, apixaban also showed a moderate half-life with t1/2 of 5.8 hours and good oral bioavailability (f: 58%) [1]. in the arteriovenous-shunt thrombosis (avst), venous thrombosis (vt) and electrically mediated carotid arterial thrombosis (ecat) rabbit models, apixaban produced antithrombotic effects with ec50 of 270 nm, 110 nm and 70 nm in a dose-dependent manner[3]. apixaban significantly inhibited factor xa activity with an ic50 of 0.22 μm in rabbit ex vivo[4]. in chimpanzee, apixaban also showed small volume of distribution (vdss: 0.17 l kg-1), low systemic clearance (cl: 0.018 l kg-1h-1), and good oral bioavailability (f: 59%) [5].
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: increased risk of haemorrhage with IV
diclofenac and ketorolac - avoid.
Antibacterials: avoid with clarithromycin and
telithromycin; concentration possibly reduced by
rifampicin - avoid if treating DVT/PE.
Anticoagulants: increased risk of haemorrhage with
other anticoagulants - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid if treating DVT/PE.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone - avoid if treating DVT/
PE with carbamazepine.
Antifungals: concentration increased by ketoconazole
- avoid; avoid with itraconazole, posaconazole and
voriconazole.
Antivirals: avoid with atazanavir, boceprevir,
darunavir, fosamprenavir, indinavir, lopinavir,
ritonavir, saquinavir, telaprevir and tipranavir.
Cobicistat: avoid concomitant use.
Metabolism
Apixaban is metabolised in the liver mainly via the P450
cytochromes CYP3A4 and CYP3A5.
Apixaban has multiple routes of elimination. Of the
administered apixaban dose in humans, approximately
25% was recovered as metabolites, with the majority
recovered in faeces. Renal excretion of apixaban accounts
for approximately 27% of total clearance. There are also
additional contributions from biliary and direct intestinal
excretion.
References
https://en.wikipedia.org/wiki/Apixaban
https://www.drugbank.ca/drugs/DB06605
Check Digit Verification of cas no
The CAS Registry Mumber 503612-47-3 includes 9 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 6 digits, 5,0,3,6,1 and 2 respectively; the second part has 2 digits, 4 and 7 respectively.
Calculate Digit Verification of CAS Registry Number 503612-47:
(8*5)+(7*0)+(6*3)+(5*6)+(4*1)+(3*2)+(2*4)+(1*7)=113
113 % 10 = 3
So 503612-47-3 is a valid CAS Registry Number.
InChI:InChI=1/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32)