
Journal of Organic Chemistry p. 787 - 802 (2016)
Update date:2022-08-15
Topics: Alkyl Aryl Alkynyl Copper-catalyzed coupling Aluminum reagents Organohalides
 Shrestha, Bijay
Shrestha, Bijay
 Thapa, Surendra
Thapa, Surendra
 Gurung, Santosh K.
Gurung, Santosh K.
 Pike, Ryan A. S.
Pike, Ryan A. S.
 Giri, Ramesh
Giri, Ramesh
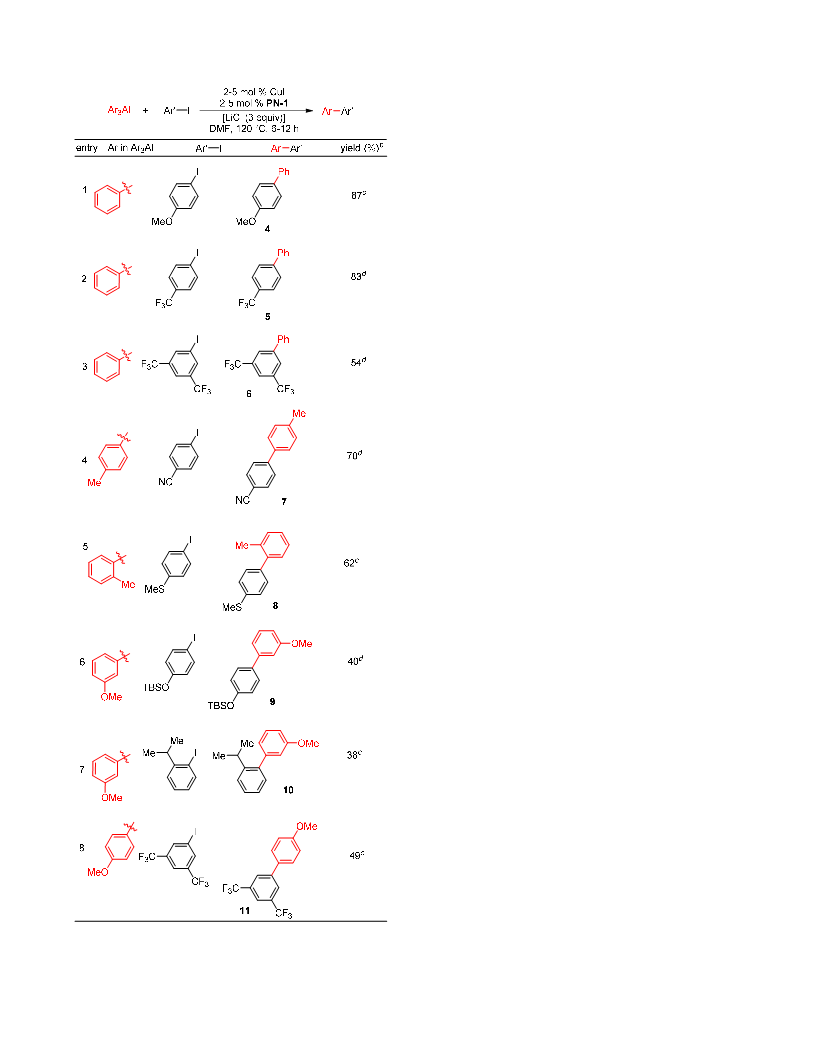
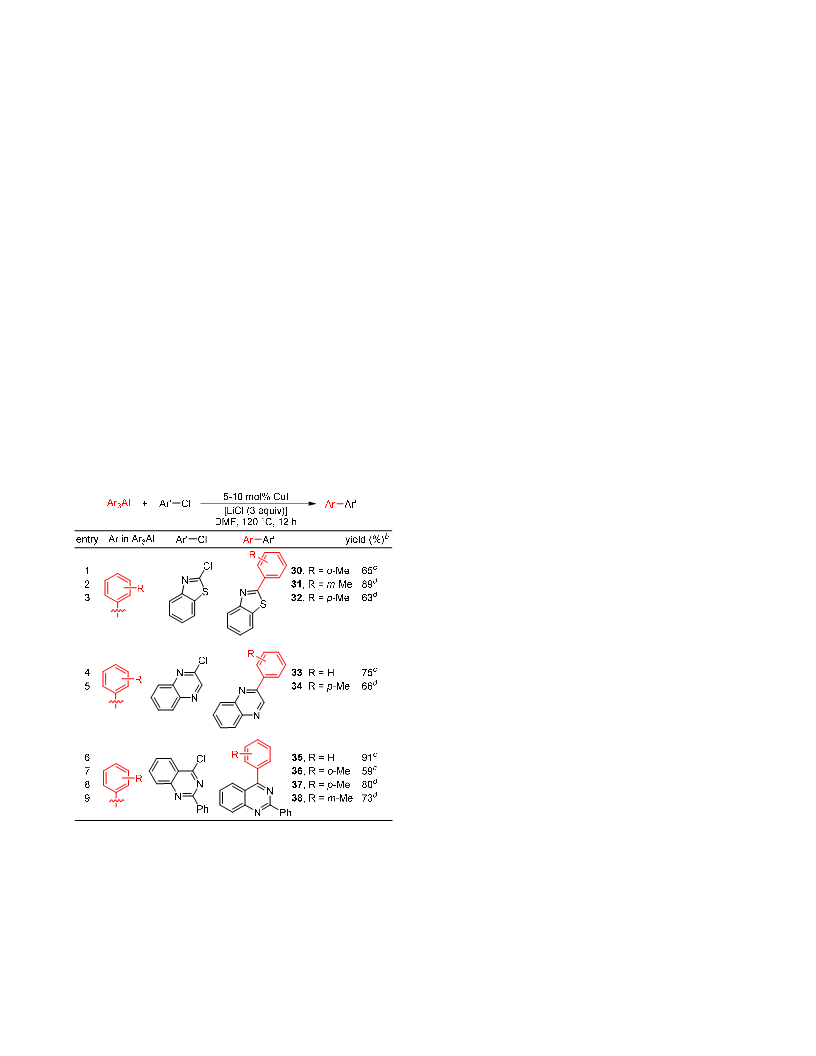
We report the first example of a very general Cu-catalyzed cross-coupling of organoaluminum reagents with organohalides. The reactions proceed for the couplings of alkyl-, aryl-, and alkynylaluminum reagents with aryl and heteroaryl halides and vinyl bromides, affording the cross-coupled products in good to excellent yields. Both primary and secondary alkylaluminum reagents can be utilized as organometallic coupling partners. These reactions are not complicated by β-hydride elimination, and as a result rearranged products are not observed with secondary alkylaluminum reagents even for couplings with heteroaryl halides under "ligand-free" conditions. Radical clock experiment with a radical probe and relative reactivity study of Ph3Al with two haloarenes, 1-bromonaphthalene and 4-chlorobenzonitrile, having two different redox potentials indicates that the reaction does not involve free aryl radicals and radical anions as intermediates. These results combined with the result of the Hammett plot obtained by reacting Ph3Al with iodoarenes containing p-H, p-Me, p-F, and p-CF3 substituents, which shows a linear curve (R2 = 0.99) with a ρ value of +1.06, suggest that the current transformation follows an oxidative addition-reductive elimination pathway.
View More















Contact:+86 18616952870
Address:Area
SPRING CHEMICAL INDUSTRY CO.,LTD
Contact:86-187-66672125
Address:linchi industry park.zouping
Contact:+86+21-58956006 15800617331
Address:402 Room, 150# Cailun Road, Zhangjiang high tech park, Shanghai
website:http://www.cheminn.com/
Contact:86-531-67875205
Address:No.9-2,South of Shanda Road, Jinan ,China
zhenjiang runjing high purity chemical co ltd
Contact:+86-511-83361155
Address:No.8.haixi road,international chemical park
Doi:10.1016/j.dyepig.2020.108182
(2020)Doi:10.1021/ol000199k
(2000)Doi:10.1023/A:1015355729220
(2002)Doi:10.1021/ja01111a027
(1953)Doi:10.1021/jm00300a009
(1970)Doi:10.1021/jo000754w
(2000)