
Journal of Organic Chemistry p. 1465 - 1468 (1987)
Update date:2022-08-16
Topics:
 Gompel, Joseph Van
Gompel, Joseph Van
 Schuster, Gary B.
Schuster, Gary B.
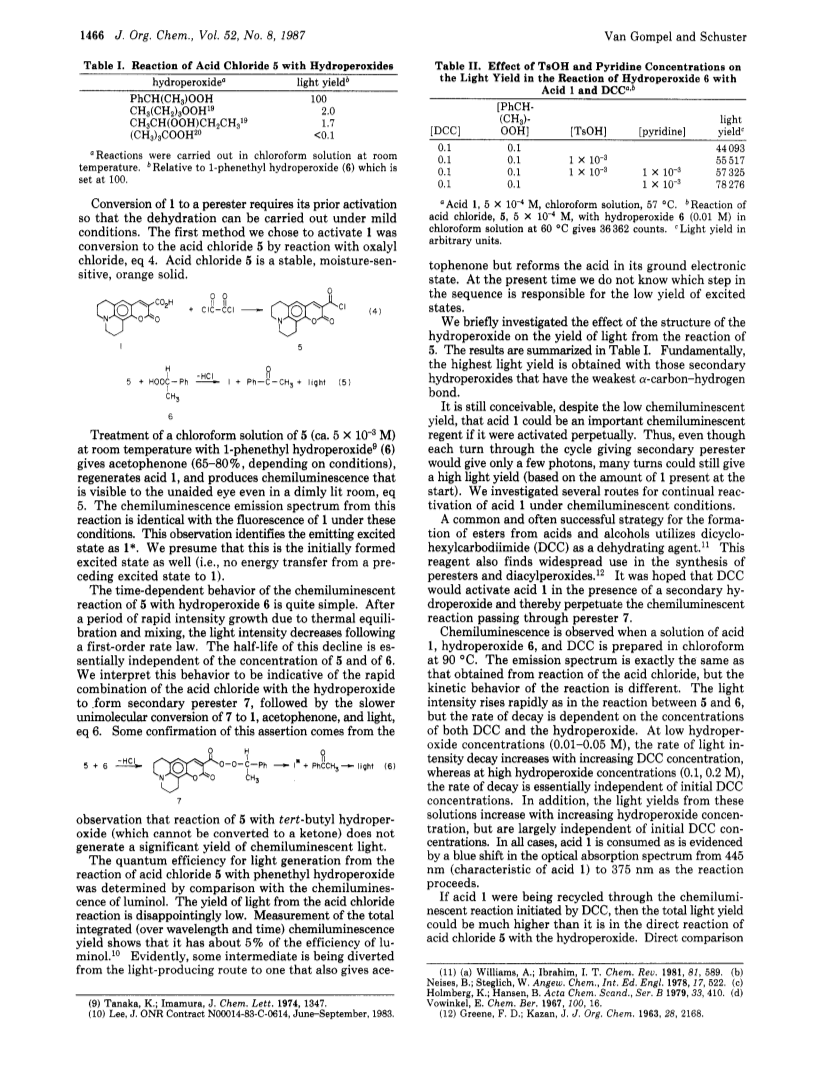
The reaction of an aminocoumarincarboxylic acid chloride (Coumarin 343) with 1-phenethyl hydroperoxide results in light emission that is easily detected with the unaided eye.This reaction proceeds through a secondary perester intermediate.Intramolecular electron exchange, modeled after the analogous process in the bioluminescence of the firefly, is proposed as the mechanism for light generation.Attempts to carry out this reaction under conditions where the coumarin acid is recycled were not successful.
View More


Shenzhen Feiming Science and Technology Co,. Ltd
Contact:+86-755-85232577
Address:#B2309, Fenglin International Center ,Jixiang Road, Longcheng street, LongGang District, Shenzhen city, Guangdong province, China.
Contact:86-513-84128750/13773795976
Address:No.48.Youyi West Road ,Rudong Development Zone,Jiangsu Province,China
HANGZHOU EVC CHEMICAL CO.,LTD.(expird)
Contact:+86-571-88296056
Address:Room#3001,Zhejiang Chemical Market,No.10,Road Shenban,Hangzhou,Zhejiang,China
Jiangsu Dacheng Pharmaceutical and Chemical Co.,Ltd
Contact:+86-0517-87036900
Address:Chuzhou Chemical park, Huai'an, Jiangsu Province
Shandong Yaroma Perfumery Co., Ltd.
Contact:+86- 531- 88024598
Address:7-702 Caizhi Central, 59 Gong Ye South Road, Jinan City,250101, P. R. China
Doi:10.1016/j.bmcl.2017.07.057
(2017)Doi:10.1016/j.ejmech.2020.112113
(2020)Doi:10.1063/1.442252
(1981)Doi:10.1021/ja00395a051
(1981)Doi:10.1016/S0040-4020(01)87062-9
(1991)Doi:10.1002/cmdc.201300277
(2013)