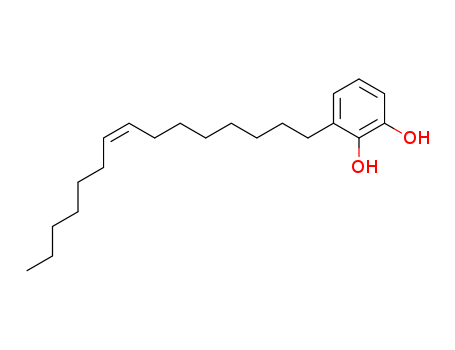
- Chemical Name:Urushiol II
- CAS No.:35237-02-6
- Molecular Formula:C21H34O2
- Molecular Weight:318.5
- Hs Code.:
- European Community (EC) Number:683-163-6
- UNII:6ZV92GML86
- DSSTox Substance ID:DTXSID001347754
- Metabolomics Workbench ID:75849
- Nikkaji Number:J1.862.328E
- Wikidata:Q27265793
- Mol file:35237-02-6.mol
Synonyms:Urushiol II;35237-02-6;Urushiol II [MI];Urushiol, (15:1;8Z);(15:1)-urushiol;UNII-6ZV92GML86;6ZV92GML86;3-[(Z)-pentadec-8-enyl]benzene-1,2-diol;1,2-Benzenediol, 3-(8Z)-8-pentadecen-1-yl-;3-(8Z-pentadecenyl)-catechol;(Z)-3-(pentadec-8-en-1-yl)benzene-1,2-diol;Bhilawanol A;Urushiol (15:1);Urushiol II (C15:1);SCHEMBL10532605;DTXSID001347754;(15:1)-URUSHIOL [MI];LMPK15020002;AKOS040735152;NS00094545;1,2-dihydroxy-3-((z)-pentadec-8-enyl)benzene;Q27265793