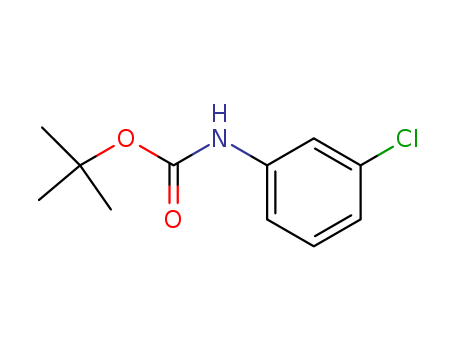
- Chemical Name:ert-butyl N-(3-chlorophenyl)carbamate
- CAS No.:5330-63-2
- Molecular Formula:C11H14ClNO2
- Molecular Weight:227.691
- Hs Code.:2924299090
- Mol file:5330-63-2.mol
Synonyms:3-chloro-NHBoc-aniline;3-Cl-Ph-NH2;(3-chlorophenyl)-carbamic acid tert-butyl ester;tert-butyl(3-chlorophenyl)carbamate;N-(tert-butoxycarbonyl)-3-chloroaniline;