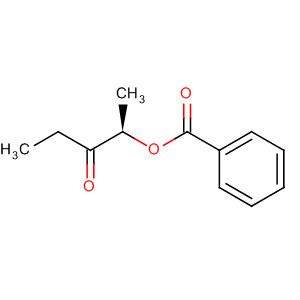
- Chemical Name:(R)-3-Oxopentan-2-yl benzoate
- CAS No.:460997-47-1
- Molecular Formula:C12H14O3
- Molecular Weight:206.241
- Hs Code.:
- DSSTox Substance ID:DTXSID50465423
- Nikkaji Number:J1.764.893D
- Wikidata:Q82291359
- Mol file:460997-47-1.mol
Synonyms:(R)-3-Oxopentan-2-yl benzoate;460997-47-1;[(2R)-3-oxopentan-2-yl] Benzoate;(2R)-2-(benzoyloxy)-3-Pentanone;(R)-3-Oxopentan-2-ylbenzoate;DTXSID50465423;(2R)-3-Oxopentan-2-yl benzoate;AKOS022173054;A23114