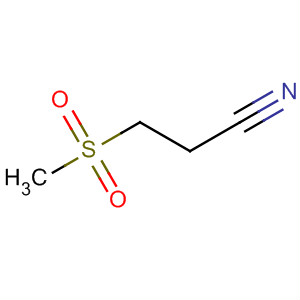
- Chemical Name:Dapansutrile
- CAS No.:54863-37-5
- Molecular Formula:C4H7NO2S
- Molecular Weight:
- Hs Code.:
- European Community (EC) Number:821-352-9
- UNII:2Z03364G96
- ChEMBL ID:CHEMBL3989943
- DSSTox Substance ID:DTXSID601336541
- NCI Thesaurus Code:C166608
- Wikidata:Q27255815
- Wikipedia:Dapansutrile
- Mol file:54863-37-5.mol
Synonyms:3-(methylsulfonyl)propanenitrile;dapansutrile;OLT-1177;OLT117;OLT1177