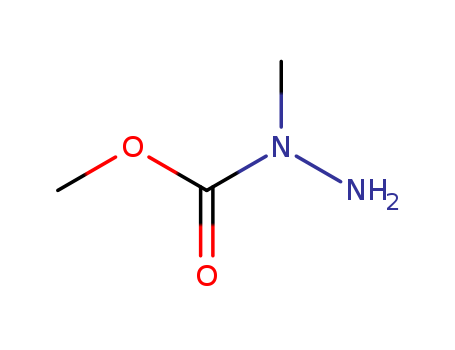
- Chemical Name:hydrazinecarboxylic acid, 1-methyl-, methyl ester
- CAS No.:20628-41-5
- Molecular Formula:C3H8N2O2
- Molecular Weight:104.109
- Hs Code.:2928000090
- Mol file:20628-41-5.mol
Synonyms:2-Methyl-carbazidsaeure-methylester;N-methyl-hydrazine-carboxylic acid methyl ester;methyl N-amino-N-methyl-carbamate;N-methylmethoxycarbohydrazide;N-methyl-N-methoxy-carbonylhydrazine;2-methyl-carbazic acid methyl ester;Methyl 1-methylhydrazinecarboxylate;N-Methyl-hydrazin-N-carbonsaeuremethylester;