10.1016/S0040-4020(01)92484-6
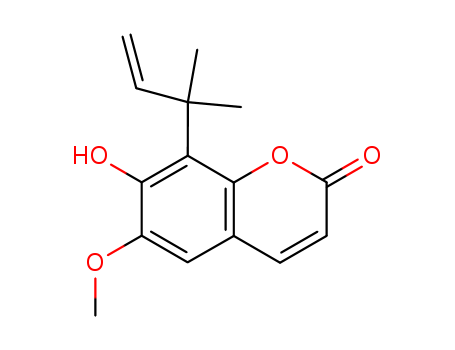
The research aims to elucidate the structures of several natural coumarins found in Ptaeroxylon obliquum (sneezewood) and synthesize them from aesculetin (5). The study confirms the structures of three coumarins (1-3) through UV, NMR, and IR spectroscopy, and demonstrates their interconversion via Claisen rearrangement. The key chemicals involved include aesculetin (5), scopoletin (6), and various derivatives such as 7-mono-ether (8) and 3,3-dimethylallyl bromide. The researchers synthesized coumarin 1 from scopoletin and used pyrolysis to produce obliquetin (2), nieshoutin (3), and an unexpected out-of-ring rearrangement product, 10. Methylation of 10 yielded rutacultin (11). The study concludes that the biogenesis of these coumarins might follow a similar pathway involving Claisen rearrangements, providing valuable insights into the natural synthesis of these compounds and their potential applications.