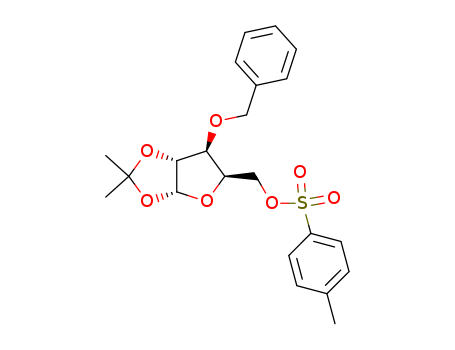
- Chemical Name:Xylofuranose, benzyl isopropylidene, toluenesulf
- CAS No.:29581-48-4
- Molecular Formula:C22H26 O7 S
- Molecular Weight:434.51
- Hs Code.:
- DSSTox Substance ID:DTXSID90952064
- Mol file:29581-48-4.mol
Synonyms:29581-48-4;Xylofuranose, benzyl isopropylidene, toluenesulf;DTXSID90952064;3-O-Benzyl-5-O-(4-methylbenzene-1-sulfonyl)-1,2-O-(1-methylethylidene)pentofuranose