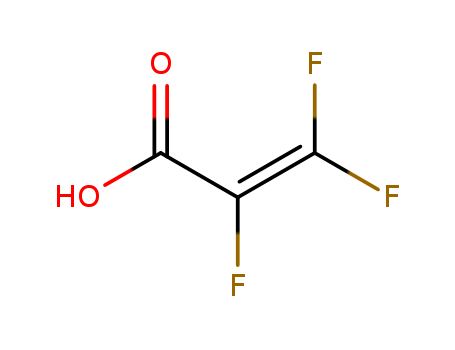
- Chemical Name:2,3,3-Trifluoroacrylic acid
- CAS No.:433-68-1
- Molecular Formula:C3H F3 O2
- Molecular Weight:126.035
- Hs Code.:
- European Community (EC) Number:207-090-2
- UNII:SZ4NS4U8T9
- DSSTox Substance ID:DTXSID70195816
- Nikkaji Number:J196.123C
- Mol file:433-68-1.mol
Synonyms:2,3,3-Trifluoroacrylic acid;433-68-1;2,3,3-trifluoroprop-2-enoic acid;SZ4NS4U8T9;EINECS 207-090-2;UNII-SZ4NS4U8T9;perfluoroacrylic acid;C3HF3O2;SCHEMBL132786;2,3,3-trifluoro-acrylic acid;2,3,3-Trifluoropropenoic acid;DTXSID70195816;C3-H-F3-O2;AKOS006380505