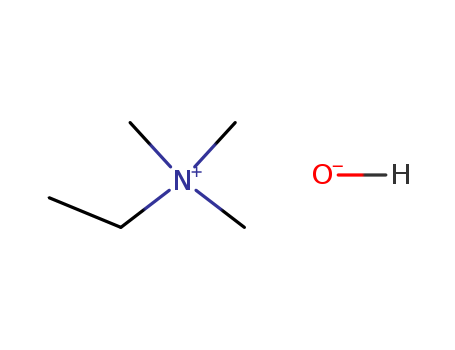
- Chemical Name:Ethanaminium, N,N,N-trimethyl-, hydroxide
- CAS No.:30382-83-3
- Molecular Formula:C5H14 N . H O
- Molecular Weight:105.18
- Hs Code.:
- European Community (EC) Number:608-473-0
- DSSTox Substance ID:DTXSID5067552
- Mol file:30382-83-3.mol
Synonyms:Ethanaminium, N,N,N-trimethyl-, hydroxide;30382-83-3;Ethyltrimethylammonium hydroxide;ethyl(trimethyl)azanium;hydroxide;Ethanaminium, N,N,N-trimethyl-, hydroxide (1:1);Trimethylathylammoniumhydroxyd;C5H14N.HO;ethyltrimethylazanium hydroxide;SCHEMBL187547;DTXSID5067552;C5-H14-N.H-O