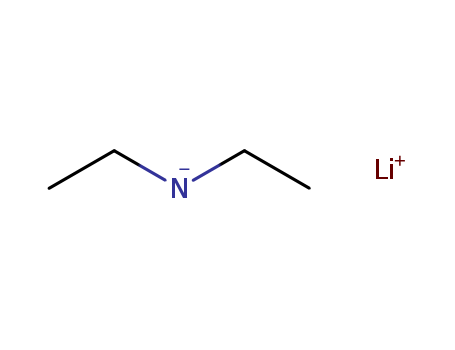
10.1055/s-2000-6323
The research aims to investigate the regioselective alkylation of lithium dienediolates derived from α,β-unsaturated carboxylic acids using tosylates, which are derived from both primary and secondary alcohols. The study focuses on improving regio- and diastereoselectivity compared to the corresponding alkylation with alkyl halides. The researchers found that the alkylation with tosylates proceeded mainly through the α-carbon, yielding high α-regioselectivities and moderate diastereoselectivities. This regioselectivity was attributed to a strong coordination of lithium ions to the oxygen atoms of the sulfonyloxy moiety, which excludes the amine and influences the transition state. The chemicals used in the process include a variety of α,β-unsaturated carboxylic acids, tosylates such as 2-tosyloxybutane, 1-tosyloxyoctane, 1-tosyloxycyclohexane, and 1-phenyl-1-tosyloxyethane, as well as lithium diethylamide (LDE) as a base. The study concludes that the nature and reactivity of the electrophile significantly affect the regioselectivity of the alkylation, and that sulphonates consistently result in regioselective α-alkylated products due to a coordinative transition state.
10.1002/anie.201805203
The study presents a novel ring transposition process for synthesizing highly substituted 2-naphthols and BINOLs using lithium bases, specifically lithium diethylamide (LiNEt2) and lithium diisopropylamide (LDA). The process involves the conversion of readily available coumarins into 2-naphthols through a series of reactions where lithium bases act as both nucleophiles and bases. Initially, the lithium bases facilitate the ring opening of coumarins to form Z-cinnamamides, which serve as in situ directing groups. These Z-cinnamamides, with their conformational freedom, undergo a directed remote metalation and ring closure reaction, yielding aryl 2-naphthols in good to excellent yields. The study also provides mechanistic insights into the remote lateral metalation step, emphasizing the necessity of Z-cinnamamide for the reaction's success. Furthermore, the methodology is applied to the synthesis of highly substituted 3,3’-diaryl BINOL ligands, which are important in enantioselective synthesis and molecular recognition. The purpose of these chemicals is to demonstrate a new synthetic strategy that can efficiently produce complex molecular structures with potential applications in natural products, dyes, pigments, and as ligands and catalysts in asymmetric synthesis.


 F
F C
C


