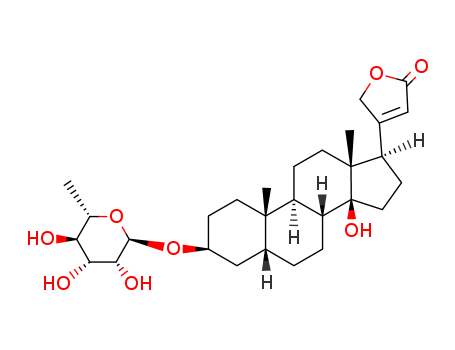
- Chemical Name:EVOMONOSIDE
- CAS No.:508-93-0
- Molecular Formula:C29H44 O8
- Molecular Weight:520.664
- Hs Code.:
- Mol file:508-93-0.mol
Synonyms:Evomonoside(6CI,7CI,8CI); Digitoxigenin 3-rhamnoside; Digitoxigenin rhamnoside; Digitoxigenin-3b-O-a-L-rhamnopyranoside