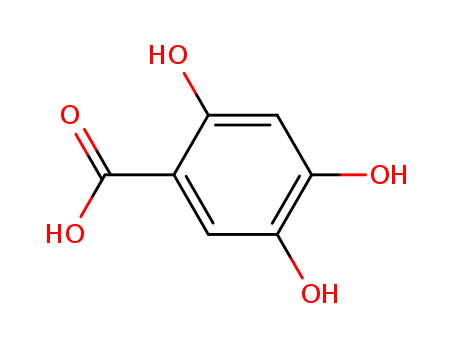
- Chemical Name:2,4,5-Trihydroxybenzoic acid
- CAS No.:610-90-2
- Molecular Formula:C7H6O5
- Molecular Weight:170.122
- Hs Code.:2918290000
- NSC Number:2813
- UNII:O9A1580JUY
- DSSTox Substance ID:DTXSID10209865
- Nikkaji Number:J1.274.532J
- Wikidata:Q27285511
- Mol file:610-90-2.mol
Synonyms:2,4,5-Trihydroxybenzoic acid;610-90-2;Benzoic acid, 2,4,5-trihydroxy-;BRN 2720657;UNII-O9A1580JUY;AI3-19304;O9A1580JUY;NSC-2813;4-10-00-01979 (Beilstein Handbook Reference);NSC2813;2,4,5-Trihydroxybenzoicacid;SCHEMBL975930;3,4,6-trihydroxybenzoic acid;DTXSID10209865;2,5-TRIHYDROXYBENZOIC ACID;NSC 2813;Benzoic acid, 2,4,5-tri-hydroxyl-;AKOS006276541;LS-38397;FT-0691407;Q27285511