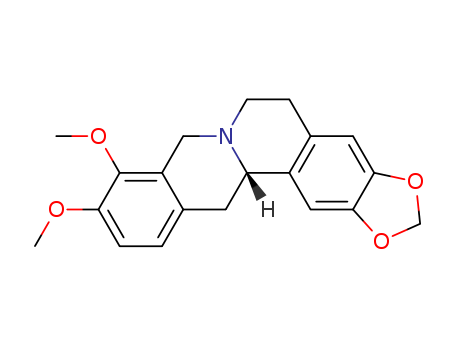
- Chemical Name:Canadine
- CAS No.:522-97-4
- Deprecated CAS:4979-14-0,26548-75-4,28111-09-3,29074-38-2,26548-75-4,28111-09-3
- Molecular Formula:C20H21NO4
- Molecular Weight:339.391
- Hs Code.:
- European Community (EC) Number:208-338-2
- NSC Number:94918,36351
- UNII:V2SSH085X8
- DSSTox Substance ID:DTXSID5022724
- Nikkaji Number:J252.482A
- Wikipedia:Canadine
- Wikidata:Q27109653
- Pharos Ligand ID:29KSTHZTLRHM
- Metabolomics Workbench ID:129996
- ChEMBL ID:CHEMBL275097
- Mol file:522-97-4.mol
Synonyms:canadine;canadine hydrochloride;canadine, (+-)-isomer;canadine, (R)-isomer;canadine, (S)-isomer;tetrahydroberberine


 Xn
Xn


