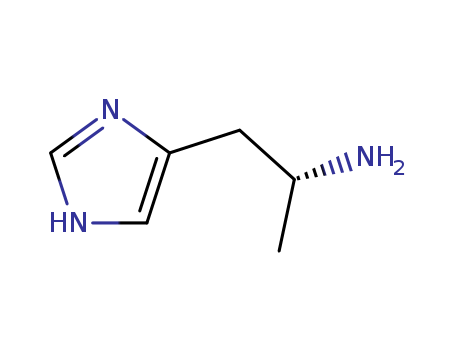
- Chemical Name:(R)-alpha-Methylhistamine
- CAS No.:75614-87-8
- Molecular Formula:C6H11 N3
- Molecular Weight:125.173
- Hs Code.:29332900
- DSSTox Substance ID:DTXSID00873372
- Nikkaji Number:J139.260C
- Wikidata:Q72491200
- Pharos Ligand ID:G3Y8FBPK34HD
- Metabolomics Workbench ID:66370
- ChEMBL ID:CHEMBL268229
- Mol file:75614-87-8.mol
Synonyms:(R)-alpha-Methylhistamine;75614-87-8;(2R)-1-(1H-imidazol-5-yl)propan-2-amine;CHEBI:73337;r-alpha-methylhistamine;(R)alpha-Me-histamine;[3H]-R-alpha-Methylhistamine;CHEMBL268229;(R)-[3H]alpha-methylhistamine;[3H](R)-alpha-methylhistamine;(2R)-1-(1H-imidazol-4-yl)propan-2-amine;(R)-(-)-alpha-methylhistamine;R(-)-alpha-Methylhistamine.2HBr;1H-Imidazole-4-ethanamine, alpha-methyl-, (alphaR)-;(R)-(-)-4-(2-aminopropyl)imidazole;(R)-1-(1H-Imidazol-5-yl)propan-2-amine;(2R)-1-(3H-imidazol-4-yl)propan-2-amine;Alpha-Methylhistamine-R;C6H11N3;Alpha Methylhistamine;(R)-alpha-MeHA;RAMH;Alpha-Methylhistane-R;Tocris-0569;R(-)-|A-Methyl Histamine Dihydrochloride;r(-)alpha methylhistamine;D03SAM;D04HEZ;R-2-(1H-Imidazol-4-yl)-1-methyl-ethylamine;Lopac0_000618;SCHEMBL73663;GTPL1236;GTPL1237;SCHEMBL4155130;BDBM22904;DTXSID00873372;alpha-methylhistamine, (R)-isomer;BDBM50215536;PDSP1_000535;PDSP1_000541;PDSP1_000542;PDSP2_000507;PDSP2_000533;PDSP2_000539;AKOS006343508;CCG-204707;SDCCGSBI-0050600.P002;NCGC00024656-01;NCGC00024656-02;NCGC00024656-03;NCGC00024656-04;NCGC00024656-08;LS-177199;LS-191796;alpha-methylhistamine-dihydrobromide-(R)-(-);(R)-2-(3H-Imidazol-4-yl)-1-methyl-ethylamine;EN300-1817458