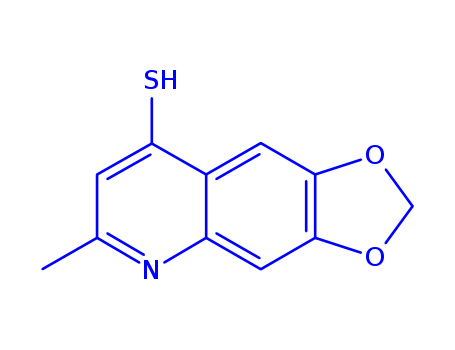
- Chemical Name:6-Methyl(1,3)dioxolo(4,5-g)quinolin-8-yl hydrosulfide
- CAS No.:91918-89-7
- Molecular Formula:C11H9NO2S
- Molecular Weight:219.264
- Hs Code.:
- NSC Number:332382
- UNII:AK4VC2XJK7
- DSSTox Substance ID:DTXSID80238788
- Mol file:91918-89-7.mol
Synonyms:91918-89-7;AK4VC2XJK7;6-Methyl(1,3)dioxolo(4,5-g)quinolin-8-yl hydrosulfide;UNII-AK4VC2XJK7;NSC332382;NSC 332382;NSC-332382;6-Methyl[1,3]dioxolo[4,5-g]quinolin-8-yl hydrosulfide;DTXSID80238788;6-Methyl[1,3]dioxolo[4,5-g]quinoline-8-thiol;6-methyl-[1,3]dioxolo[4,5-g]quinoline-8-thiol;6-METHYL-1,3-DIOXOLO(4,5-G)QUINOLINE-8-THIOL;1,3-DIOXOLO(4,5-G)QUINOLINE-8-THIOL, 6-METHYL-