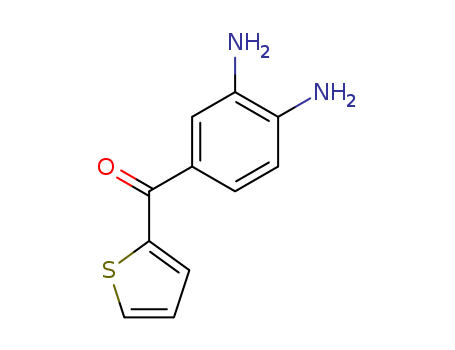
- Chemical Name:(3,4-Diaminophenyl) (2-thienyl) ketone
- CAS No.:83846-78-0
- Molecular Formula:C11H10 N2 O S
- Molecular Weight:218.279
- Hs Code.:2934999090
- European Community (EC) Number:281-057-0
- DSSTox Substance ID:DTXSID20232745
- Nikkaji Number:J264.941A
- Wikidata:Q83113873
- Mol file:83846-78-0.mol
Synonyms:83846-78-0;(3,4-Diaminophenyl) (2-thienyl) ketone;(3,4-diaminophenyl)-thiophen-2-ylmethanone;EINECS 281-057-0;(3,4-Diaminophenyl)thiophen-2-ylmethanone;SCHEMBL8291933;DTXSID20232745;(3,4-Diaminophenyl)(2-thienyl)ketone;(3,4-Diaminophenyl)(2-thienyl)methanone #;Methanone, (3,4-diaminophenyl)-2-thienyl-