10.1021/jm800967h
The research presents the design and synthesis of novel tricyclic [1,2,4]triazine 1,4-dioxides (TTOs), which are hypoxia-selective cytotoxins related to tirapazamine. The purpose of this study was to develop TTO analogues that display hypoxia-selective cytotoxicity in vitro, with the aim of improving the treatment of hypoxic tumors, which are associated with resistance to therapy and aggressive tumor behavior. The researchers synthesized a series of TTOs with various structural arrangements, including cycloalkyl, oxygen-, and nitrogen-containing saturated rings fused to the triazine core, along with different side chains linked to either hemisphere. They optimized the rates of hypoxic metabolism and tissue diffusion coefficients, and used pharmacokinetic/pharmacodynamic model predictions to identify 12 TTO analogues predicted to be active in vivo, contingent upon achieving adequate plasma pharmacokinetics. The chemicals used in the process included a range of amines, cyanamide, peracetic acid, trifluoroacetic acid, and various nitroanilines and their derivatives, among others, to construct the diverse TTO analogues. The study concluded that the fused cycloalkyl rings, in combination with 3-aminoalkyl or 3-alkyl substituents linked to weakly basic soluble amines, resulted in TTOs with optimal hypoxic metabolism and diffusion coefficients, highlighting the potential of these compounds as clinical agents that selectively target hypoxia in tumors.

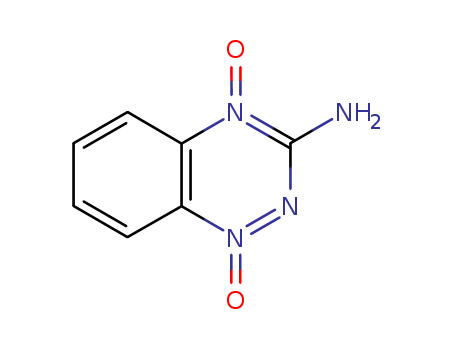

 Xi
Xi

