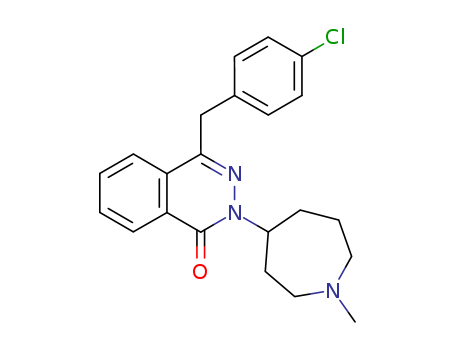
- Chemical Name:Azelastine
- CAS No.:58581-89-8
- Deprecated CAS:153483-42-2
- Molecular Formula:C22H24ClN3O
- Molecular Weight:381.905
- Hs Code.:2933990090
- European Community (EC) Number:611-699-2
- UNII:ZQI909440X
- DSSTox Substance ID:DTXSID6022638
- Nikkaji Number:J13.831B
- Wikipedia:Azelastine
- Wikidata:Q419820
- NCI Thesaurus Code:C61643
- RXCUI:18603
- Pharos Ligand ID:VKY49BVV56A6
- Metabolomics Workbench ID:145700
- ChEMBL ID:CHEMBL639
- Mol file:58581-89-8.mol
Synonyms:4-((4-chlorophenyl)methyl)-2- (hexahydro-1-methyl-1H-azepin-4-yl)-1(2H)- phthalazinone HCl;4-(p-chlorobenzyl)-2-(N-methylperhydroazepinyl-(4))-1-(2H)-phthalazinone;A 5610;A-5610;Afluon;Allergodil;Astelin;azelastine;azelastine hydrochloride;Azeptin;Corifina;Loxin;Optilast;Optivar;Rhinolast;Vividrin akut Azelastin


 N,
N, T
T


