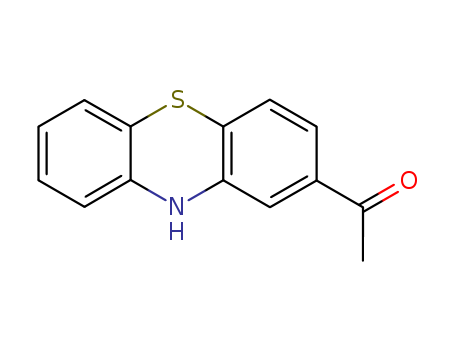
- Chemical Name:1-(10H-Phenothiazin-2-yl)ethanone
- CAS No.:6631-94-3
- Molecular Formula:C14H11 N O S
- Molecular Weight:241.313
- Hs Code.:2934300000
- European Community (EC) Number:229-626-4
- NSC Number:169669,57951
- UNII:8DEE7H3XAB
- DSSTox Substance ID:DTXSID20216525
- Nikkaji Number:J124.130C
- Wikidata:Q27164209
- Pharos Ligand ID:JG7A3VFLU18H
- ChEMBL ID:CHEMBL407734
- Mol file:6631-94-3.mol
Synonyms:1-(10H-phenothiazin-2-yl)ethanone