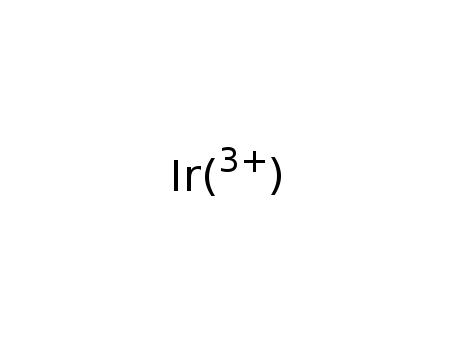- Chemical Name:Iridium (III) ion
- CAS No.:22555-00-6
- Molecular Formula:Ir
- Molecular Weight:192.22
- Hs Code.:
- DSSTox Substance ID:DTXSID70177091
- Wikidata:Q27121632
- Mol file:22555-00-6.mol
Synonyms:iridium(3+);IRIDIUM (III) ION;Iridium, ion(Ir3 );22555-00-6;IR3;IRIDIUM ION;Iridium, ion (Ir3+);Ir(III);CHEBI:49704;DTXSID70177091;Q27121632