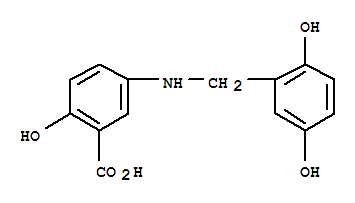
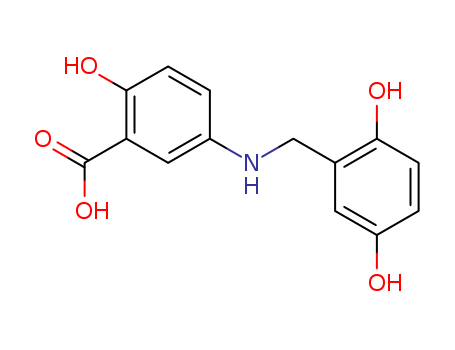
- Chemical Name:Lavendustin c
- CAS No.:125697-93-0
- Molecular Formula:C14H13 N O5
- Molecular Weight:275.261
- Hs Code.:2922509090
- European Community (EC) Number:635-029-3
- NSC Number:666251
- UNII:HJM06BIW5M
- DSSTox Substance ID:DTXSID60154856
- Nikkaji Number:J568.039E
- Wikidata:Q27166541
- NCI Thesaurus Code:C1487
- Metabolomics Workbench ID:130711
- ChEMBL ID:CHEMBL319620
- Mol file:125697-93-0.mol
Synonyms:2-hydroxy-5-(2,5-dihydrobenzyl) aminobenzoic acid;2-hydroxy-5-(2,5-dihydrobenzyl)aminobenzoic acid;HBABA


 Xi
Xi

