10.1055/s-0028-1083601
The research focuses on the development and synthesis of chemically modified linear and cyclic polyglycerols and their esters, specifically triglyceryl di-, tri-, and tetra-fatty acid esters, which are structurally well-defined and have a single polymerization degree. The purpose of this study is to create new oil gelators capable of gelling up cooking oils, with a particular emphasis on understanding the role of alkyl chain length in the gelation process. The study concluded that these triglyceryl ester gelators exhibit prominent gelation ability and can gel a wide variety of oils. Key chemicals used in the synthesis process include triglycerol, fatty acid esters such as stearic, myristic, palmitic, and arachidic acids, benzyl bromide, stearoyl chloride, and various reagents for protection and deprotection steps, as well as catalysts and solvents like pyridine, 4-(dimethylamino)pyridine, and palladium on carbon for hydrogenolysis.

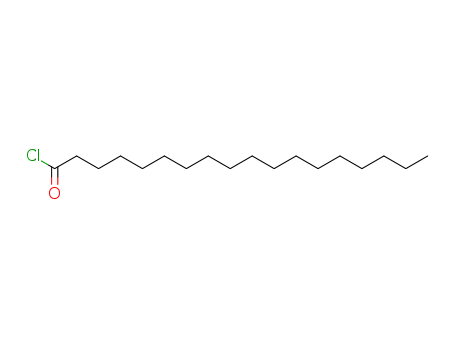

 C
C


