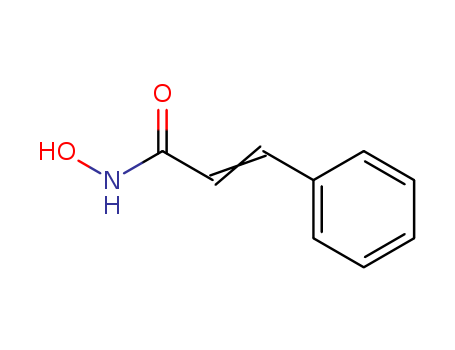
10.1021/ml300366t
The research focuses on the development of a novel series of histone deacetylase inhibitors (HDACIs) that combine an N-hydroxycinnamamide bioactive fragment with an indole bioactive fragment. Several compounds, including 17c, 17g, 17h, 17j, and 17k, exhibited comparable or even superior total HDACs inhibitory activity and in vitro antiproliferative activities relative to the approved drug SAHA. The study also delved into the isoform selectivity profile, Western blot analysis, and in vivo antitumor assay of a representative compound 17a. Key chemicals involved in the research include N-hydroxycinnamamide, indole, and various derivatives and intermediates such as compounds 14a, 14b, 15, and 17a?u. The synthesis process involved multiple steps, including methyl ester protection, tert-butyloxycarbonyl (Boc) protection, LiAlH4 reduction, hydrogenization, Mitsunobu reaction, and N-deprotection. The results indicated that certain compounds maintained or even increased their effects on histone H4 acetylation over time, suggesting potential applications in tumor prevention and treatment.