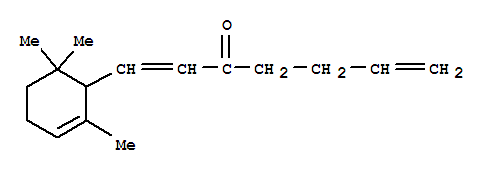10.1021/jo010963w
The research aims to investigate the photochemical transformations of (E,E)-arylidene--ionones (1a-f) and their derivatives under various conditions. The study's purpose is to correct structural assignments, establish the exo-selectivity of photocycloadditions, and discover alternative modes of phototransformation, particularly focusing on the formation of tricyclic ketones. The research concludes that these phototransformations provide a highly efficient route to complex molecular frameworks and versatile synthons, which are important in the synthesis of biologically active molecules. The chemicals used in the process include arylidene--ionones (1a-f), their corresponding pyrans (3a-f), and various aryl groups such as phenyl, p-tolyl, p-anisyl, p-bromophenyl, p-chlorophenyl, and furan-2-yl, among others. The reactions were carried out in anhydrous solvents and aqueous methanol, with irradiation playing a crucial role in the transformations.