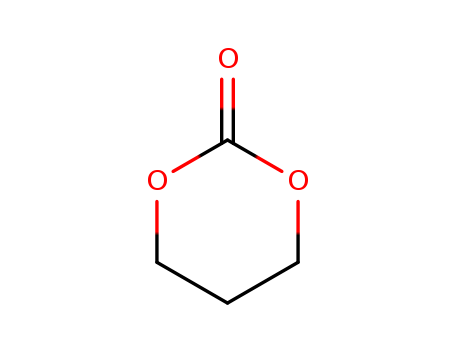
- Chemical Name:1,3-dioxan-2-one
- CAS No.:31852-84-3
- Molecular Formula:C4H6O3
- Molecular Weight:102.09
- Hs Code.:
- Mol file:31852-84-3.mol
Synonyms:Carbonicacid, cyclic trimethylene ester, polymers (8CI); 1,3-Propanediol, cycliccarbonate, polymers (8CI); 1,3-Trimethylene carbonate homopolymer;Cyclotrimethylene carbonate homopolymer; Poly(trimethylene carbonate);Trimethylene carbonate homopolymer; Trimethylene carbonate polymer