10.1007/s11172-009-0199-8
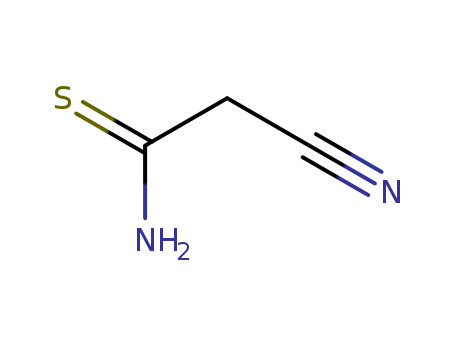
The research focuses on the Mannich reaction in the synthesis of N,S-containing heterocycles, specifically the recyclization of stable cyclic Michael adducts, N-methylmorpholinium 6-R-6-hydroxy-1,4,5,6-tetrahydropyridine-2-thiolates, to pyrimido[4,3-b][1,3,5]thiadiazines under aminomethylation reaction conditions. The experiments involve the reaction of 5-substituted N-methylmorpholinium 4-aryl-6-R-3-cyano-6-hydroxy-1,4,5,6-tetrahydro-pyridine-2-thiolates with primary amines and formaldehyde (HCHO) in ethanol, leading to the formation of 3,7-disubstituted 8-aryl-3,4,7,8-tetrahydro-2H,6H-pyrimido[4,3-b][1,3,5]thiadiazine-9-carbonitriles in low yields (5—28%). The reactants include cyanothioacetamide, aromatic aldehydes, primary amines, and HCHO. The analyses used to characterize the products include infrared (IR) spectroscopy, proton nuclear magnetic resonance (1H NMR) spectroscopy, and elemental analysis, which confirmed the structures of the synthesized pyrimido[4,3-b][1,3,5]thiadiazines.
10.1134/S1070428018090014
The research focuses on the development of a new multicomponent synthesis method for the production of 4,6-diaryl-2-sulfanylidene-1,2-dihydropyridine-3-carbonitrile derivatives, which are important heterocyclic compounds with potential applications in the synthesis of antihypertensive and antidiabetic agents. The study involves the condensation of aromatic aldehydes, acetophenones, cyanothioacetamide, and alkylating agents to yield a variety of pyridine and thienopyridine derivatives. The synthesized compounds were confirmed through X-ray analysis, spectral data, and other analytical techniques. The study concludes that this method is advantageous due to the accessibility of initial reactants, simplicity of experimental procedures, high yields, and alignment with environmental safety demands.
10.1134/S1070428007020194
The research focuses on the development of a new synthetic method for functionally substituted morpholinium 1,4-dihydropyridine-2-thiolates and their derivatives. The experiments involve the condensation of aromatic aldehydes with cyanothioacetamide and enamines of 1,3-dicarbonyl compounds to obtain these compounds, which have potential applications in the synthesis of biologically active compounds with antioxidant, hepatoprotector, and cardiovascular qualities. The reactants used in the synthesis include various aromatic aldehydes, cyanothioacetamide, and enamines of 1,3-dicarbonyl compounds. The structures of the synthesized compounds were confirmed through spectral analyses, including IR and 1H NMR spectroscopy, which provided characteristic absorption bands for the stretching vibrations of conjugated cyano and carbonyl groups, as well as proton signals from the 1,4-dihydropyridine ring. Additionally, mass spectrometry and elemental analysis were employed to further validate the structures and compositions of the synthesized compounds.


 Xn
Xn


