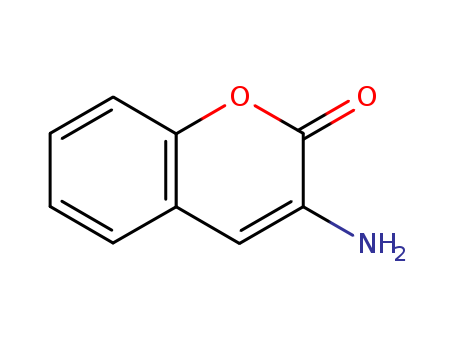
- Chemical Name:3-Aminocoumarin
- CAS No.:1635-31-0
- Molecular Formula:C9H7NO2
- Molecular Weight:161.16
- Hs Code.:29322010
- European Community (EC) Number:216-659-4
- UNII:PC6801C293
- DSSTox Substance ID:DTXSID60167603
- Nikkaji Number:J80.269G
- Wikidata:Q72435031
- Metabolomics Workbench ID:73964
- ChEMBL ID:CHEMBL1312108
- Mol file:1635-31-0.mol
Synonyms:3-Aminocoumarin;3-amino-2H-chromen-2-one;1635-31-0;3-aminochromen-2-one;3-Amino-2-benzopyrone;3-Amino-2H-1-benzopyran-2-one;EINECS 216-659-4;2H-1-benzopyran-2-one, 3-amino-;PC6801C293;coumarin amine;MLS000532598;coumarin monoamine;3-azanylchromen-2-one;3-Aminocoumarin, 97%;Oprea1_066898;SCHEMBL95179;3-amino-1-benzopyran-2-one;CHEMBL1312108;UNII-PC6801C293;DTXSID60167603;3-amino-2-oxo-2H-1-benzopyran;3-AMINO-1,2-BENZOPYRONE;HMS2178H11;AMY15166;BCP20024;MFCD00016965;STK364532;AKOS001684142;NCGC00245539-01;SMR000137537;TS-02139;FT-0615035;EN300-235650;A810483;AN-829/06063010;W-201485;F2135-0511


 Xi
Xi

