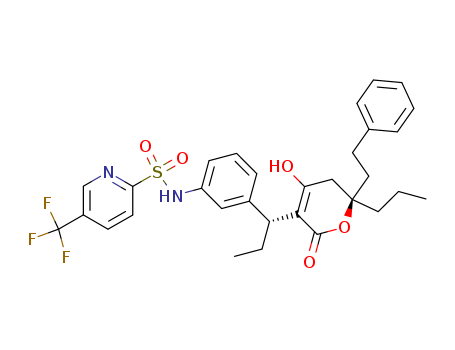
- Chemical Name:Tipranavir
- CAS No.:174484-41-4
- Molecular Formula:C31H33F3N2O5S
- Molecular Weight:602.675
- Hs Code.:
- Mol file:174484-41-4.mol
Synonyms:2-Pyridinesulfonamide,N-[3-[1-[5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyran-3-yl]propyl]phenyl]-5-(trifluoromethyl)-,[R-(R*,R*)]-;Aptivus;PNU 140690;Tipranavir;U 140690;