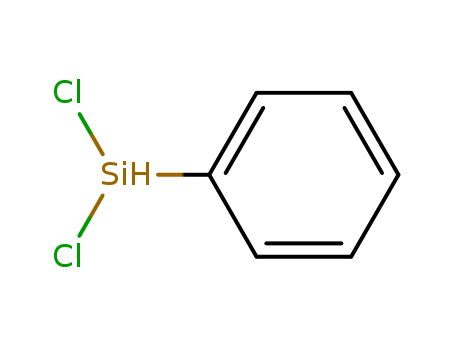
- Chemical Name:Phenyldichlorosilane
- CAS No.:1631-84-1
- Molecular Formula:C6H6Cl2Si
- Molecular Weight:177.105
- Hs Code.:2931900090
- European Community (EC) Number:216-635-3
- UNII:GT7Y64S7UH
- DSSTox Substance ID:DTXSID50870896
- Mol file:1631-84-1.mol
Synonyms:Phenyldichlorosilane;Dichlorophenylsilane;1631-84-1;Silane, dichlorophenyl-;Benzene, (dichlorosilyl)-;dichloro(phenyl)silicon;NSC 139846;GT7Y64S7UH;Phenyl(hydrogen)dichlorosilane;EINECS 216-635-3;MFCD00039296;C6H6Cl2Si;Silane, dichloro-phenyl-,;UNII-GT7Y64S7UH;[Si](Cl)(Cl)c1ccccc1;C6-H6-Cl2-Si;bis(chloranyl)-phenyl-silicon;SCHEMBL203456;DTXSID50870896;AKOS006223925;AKOS015915856;FS-5393;FT-0624726;S13350;A810451;J-010017



 C
C


