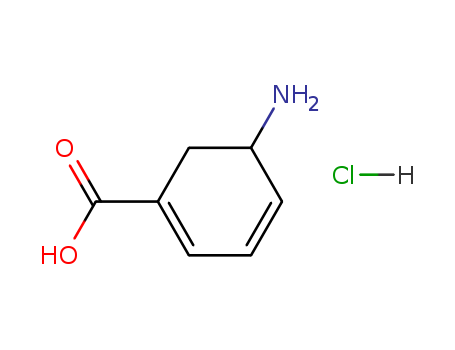
- Chemical Name:3-Amino-2,3-dihydrobenzoic acid hydrochloride
- CAS No.:59556-17-1
- Molecular Formula:C7H9 N O2 . Cl H
- Molecular Weight:175.615
- Hs Code.:2922499990
- European Community (EC) Number:261-802-6
- UNII:C3VM79824Q
- DSSTox Substance ID:DTXSID10974965
- ChEMBL ID:CHEMBL1256019
- Mol file:59556-17-1.mol
Synonyms:3-amino-2,3-dihydrobenzoic acid;gabaculin;gabaculine;gabaculine hydrochloride;gabaculine hydrochloride, (+-)-isomer;gabaculine, (+-)-isomer;gabaculine, (-)-isomer