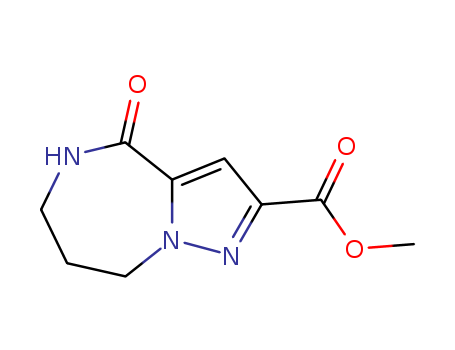
- Chemical Name:methyl 4-oxo-5,6,7,8-tetrahydro-4H-pyrazolo[1,5-a][1,4]diazepine-2-carboxylate
- CAS No.:163213-38-5
- Molecular Formula:C9H11N3O3
- Molecular Weight:209.205
- Hs Code.:2933199090
- European Community (EC) Number:692-856-2
- DSSTox Substance ID:DTXSID60377629
- Nikkaji Number:J1.921.136C
- Wikidata:Q82166913
- ChEMBL ID:CHEMBL2134607
- Mol file:163213-38-5.mol
Synonyms:163213-38-5;methyl 4-oxo-5,6,7,8-tetrahydro-4H-pyrazolo[1,5-a][1,4]diazepine-2-carboxylate;methyl 4-oxo-4H,5H,6H,7H,8H-pyrazolo[1,5-a][1,4]diazepine-2-carboxylate;methyl 4-oxo-5,6,7,8-tetrahydropyrazolo[1,5-a][1,4]diazepine-2-carboxylate;Bionet2_000392;Oprea1_362601;MLS000721518;SCHEMBL3127245;CHEMBL2134607;DTXSID60377629;VWXQPZYUOQXYLQ-UHFFFAOYSA-N;HMS1365B18;HMS2679P10;AMY35347;MFCD02570753;AKOS005078913;SB40735;Methyl4-oxo-5,6,7,8-tetrahydro-4H-pyrazolo[1,5-a][1,4]diazepine-2-carboxylate;SMR000335833;CS-0049024;F11976;11P-243;J-522386;4-Oxo-5,6,7,8-tetrahydro-4H-1,5,8a-triazaazulene-2-carboxylic acid methyl ester;Methyl 4-oxo-4H,5H,6H,7H,8H-pyrazolo-[1,5-a][1,4]diazepine-2-carboxylate;4H-Pyrazolo[1,5-a][1,4]diazepine-2-carboxylic acid, 5,6,7,8-tetrahydro-4-oxo-, methyl ester