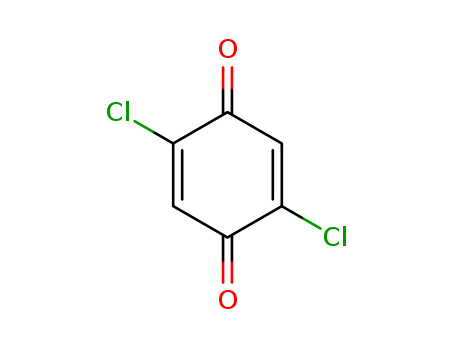
- Chemical Name:2,5-Dichloro-1,4-benzoquinone
- CAS No.:615-93-0
- Deprecated CAS:78844-56-1
- Molecular Formula:C6H2Cl2O2
- Molecular Weight:176.987
- Hs Code.:2914700090
- European Community (EC) Number:210-453-8
- NSC Number:6251
- DSSTox Substance ID:DTXSID9060657
- Nikkaji Number:J65.481G
- Wikidata:Q69759030
- ChEMBL ID:CHEMBL3311439
- Mol file:615-93-0.mol
Synonyms:2,5-DCBQ;2,5-dichlorobenzoquinone


 Xi
Xi

