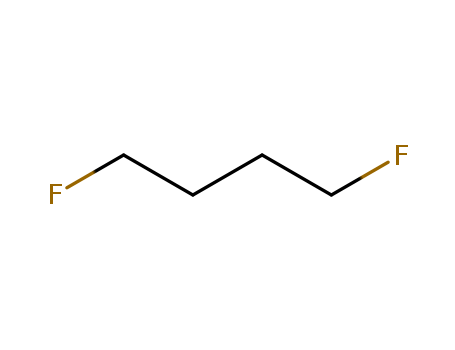
- Chemical Name:1,4-Difluorobutane
- CAS No.:372-90-7
- Molecular Formula:C4H8F2
- Molecular Weight:94.1043
- Hs Code.:2903399090
- European Community (EC) Number:671-650-6
- DSSTox Substance ID:DTXSID40190696
- Nikkaji Number:J42.715B
- Wikidata:Q18461852
- Mol file:372-90-7.mol
Synonyms:1,4-Difluorobutane;372-90-7;BUTANE, 1,4-DIFLUORO-;BRN 1731406;3-01-00-00273 (Beilstein Handbook Reference);C4H8F2;1,4-Difluorobutan;DTXSID40190696;C4-H8-F2;MFCD01740905;AKOS006227792;LS-45693;FT-0676811



 Xi
Xi


