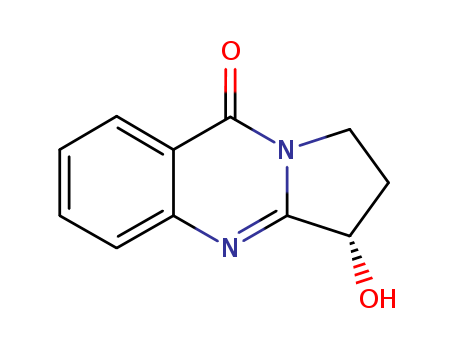
- Chemical Name:VASICINONE
- CAS No.:486-64-6
- Molecular Formula:C11H10 N2 O2
- Molecular Weight:202.213
- Hs Code.:
- European Community (EC) Number:683-100-2
- UNII:G6T5819NXM
- DSSTox Substance ID:DTXSID80964090
- Nikkaji Number:J783.610D
- Wikipedia:Vasicinone
- Wikidata:Q15427947
- Metabolomics Workbench ID:69533
- ChEMBL ID:CHEMBL3120244
- Mol file:486-64-6.mol
Synonyms:Pyrrolo[2,1-b]quinazolin-9(1H)-one,2,3-dihydro-3-hydroxy-, (S)- (8CI); Vasicinone (6CI,7CI); (-)-Vasicinone;l-Vasicinone